Fructose stimulated de novo lipogenesis is promoted by inflammation
- PMID: 32839596
- PMCID: PMC8018782
- DOI: 10.1038/s42255-020-0261-2
Fructose stimulated de novo lipogenesis is promoted by inflammation
Abstract
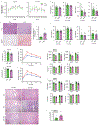
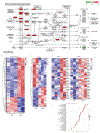
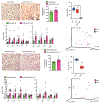
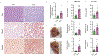
Benign hepatosteatosis, affected by lipid uptake, de novo lipogenesis and fatty acid (FA) oxidation, progresses to non-alcoholic steatohepatitis (NASH) on stress and inflammation. A key macronutrient proposed to increase hepatosteatosis and NASH risk is fructose. Excessive intake of fructose causes intestinal-barrier deterioration and endotoxaemia. However, how fructose triggers these alterations and their roles in hepatosteatosis and NASH pathogenesis remain unknown. Here we show, using mice, that microbiota-derived Toll-like receptor (TLR) agonists promote hepatosteatosis without affecting fructose-1-phosphate (F1P) and cytosolic acetyl-CoA. Activation of mucosal-regenerative gp130 signalling, administration of the YAP-induced matricellular protein CCN1 or expression of the antimicrobial peptide Reg3b (beta) peptide counteract fructose-induced barrier deterioration, which depends on endoplasmic-reticulum stress and subsequent endotoxaemia. Endotoxin engages TLR4 to trigger TNF production by liver macrophages, thereby inducing lipogenic enzymes that convert F1P and acetyl-CoA to FA in both mouse and human hepatocytes.
Conflict of interest statement
Competing Interests Statement
M.K. holds a US patent on the use of
Figures






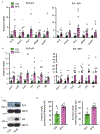


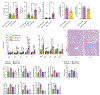
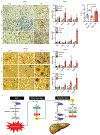
Comment in
-
Role of fructose in NASH defined.Nat Rev Endocrinol. 2020 Nov;16(11):624. doi: 10.1038/s41574-020-00419-4. Nat Rev Endocrinol. 2020. PMID: 32887949 No abstract available.
-
Altered Cholesterol and Lipid Synthesis Mediates Hyperinflammation in COVID-19.Trends Endocrinol Metab. 2021 Mar;32(3):132-134. doi: 10.1016/j.tem.2021.01.001. Epub 2021 Jan 8. Trends Endocrinol Metab. 2021. PMID: 33455862 Free PMC article.
References
-
- Stickel F & Hellerbrand C Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut 59, 1303–7 (2010). - PubMed
-
- Tilg H & Moschen AR Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52, 1836–1846 (2010). - PubMed
-
- Lebeaupin C et al. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol 69, 927–947 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK108743/DK/NIDDK NIH HHS/United States
- R03 CA223717/CA/NCI NIH HHS/United States
- R01 DK120714/DK/NIDDK NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- R01 CA211794/CA/NCI NIH HHS/United States
- S10 OD020025/OD/NIH HHS/United States
- T32 AI007469/AI/NIAID NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- R01 CA188652/CA/NCI NIH HHS/United States
- U01 AA027681/AA/NIAAA NIH HHS/United States
- R01 AI043477/AI/NIAID NIH HHS/United States
- R01 CA198103/CA/NCI NIH HHS/United States
- R01 CA192642/CA/NCI NIH HHS/United States
- R01 CA218254/CA/NCI NIH HHS/United States
- R01 ES027595/ES/NIEHS NIH HHS/United States
- K22 AI139444/AI/NIAID NIH HHS/United States
- R01 CA207177/CA/NCI NIH HHS/United States
- K01 DK116917/DK/NIDDK NIH HHS/United States
- R01 CA234128/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

