An inflammatory cytokine signature predicts COVID-19 severity and survival
- PMID: 32839624
- PMCID: PMC7869028
- DOI: 10.1038/s41591-020-1051-9
An inflammatory cytokine signature predicts COVID-19 severity and survival
Abstract
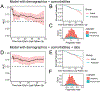
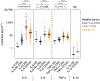
Several studies have revealed that the hyper-inflammatory response induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a major cause of disease severity and death. However, predictive biomarkers of pathogenic inflammation to help guide targetable immune pathways are critically lacking. We implemented a rapid multiplex cytokine assay to measure serum interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α and IL-1β in hospitalized patients with coronavirus disease 2019 (COVID-19) upon admission to the Mount Sinai Health System in New York. Patients (n = 1,484) were followed up to 41 d after admission (median, 8 d), and clinical information, laboratory test results and patient outcomes were collected. We found that high serum IL-6, IL-8 and TNF-α levels at the time of hospitalization were strong and independent predictors of patient survival (P < 0.0001, P = 0.0205 and P = 0.0140, respectively). Notably, when adjusting for disease severity, common laboratory inflammation markers, hypoxia and other vitals, demographics, and a range of comorbidities, IL-6 and TNF-α serum levels remained independent and significant predictors of disease severity and death. These findings were validated in a second cohort of patients (n = 231). We propose that serum IL-6 and TNF-α levels should be considered in the management and treatment of patients with COVID-19 to stratify prospective clinical trials, guide resource allocation and inform therapeutic options.
Figures
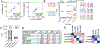



Update of
-
An inflammatory cytokine signature helps predict COVID-19 severity and death.medRxiv [Preprint]. 2020 May 30:2020.05.28.20115758. doi: 10.1101/2020.05.28.20115758. medRxiv. 2020. Update in: Nat Med. 2020 Oct;26(10):1636-1643. doi: 10.1038/s41591-020-1051-9. PMID: 32511562 Free PMC article. Updated. Preprint.
Comment in
-
COVID 19: in the eye of the cytokine storm.Eur Heart J. 2021 Jan 7;42(2):150-151. doi: 10.1093/eurheartj/ehaa1005. Eur Heart J. 2021. PMID: 33462598 Free PMC article. No abstract available.
References
-
- Paranjpe I et al. Clinical characteristics of hospitalized COVID-19 patients in New York City. Preprint at 10.1101/2020.04.19.20062117 (2020). - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

