Persistence of SARS-CoV-2 nasopharyngeal swab PCR positivity in COVID-19 convalescent plasma donors
- PMID: 32840002
- PMCID: PMC7461313
- DOI: 10.1111/trf.16015
Persistence of SARS-CoV-2 nasopharyngeal swab PCR positivity in COVID-19 convalescent plasma donors
Abstract
Background: Nucleic acid persists after symptom resolution and infectivity for many viral infections via delayed clearance of nucleic acid fragments, non-infectious particles, or transmissible virus. For Coronavirus Disease 2019 (COVID-19), the relationship between nasopharyngeal (NP) swab positivity, the development of antibodies against COVID-19, and clinical history are unclear.
Study design and methods: Individuals who recovered from COVID-19 and volunteered to donate convalescent plasma (CP) were screened by NP swab PCR, responded to a questionnaire, and were tested for anti-COVID-19 antibodies.
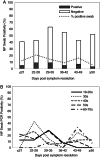
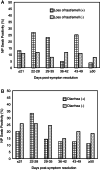
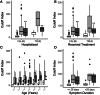
Results: A proportion of 11.8% of individuals tested positive for SARS-CoV-2 by NP swab PCR greater than 14 days after the resolution of symptoms of active disease, including one donor who had asymptomatic disease and tested positive by NP swab 41 days after her initial diagnosis. Clinical history did not show a significant correlation with persistence of NP swab positivity. Also, NP swab positivity >14 days from symptom resolution did not correlate with anti-COVID-19 serology results. IgG anti-SARS-CoV-2 spike antibody strength correlated with hospitalization for COVID-19 using two different assays. Total anti-SARS-CoV-2 nucleocapsid antibody strength correlated with time from symptom resolution to sample collection and symptom duration.
Conclusions: SARS-CoV-2 nucleic acid is detectable long after the resolution of symptoms in a significant percentage of previously diagnosed individuals, which is important to consider when interpreting PCR swab results. Persistence of PCR positivity does not correlate with antibody strength or symptoms of COVID-19. If anti-spike antibody is used to assess CP potency, individuals who suffered severe COVID-19 disease symptoms may represent better donors.
© 2020 AABB.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Impact of serological and PCR testing requirements on the selection of COVID-19 convalescent plasma donors.Transfusion. 2021 May;61(5):1461-1470. doi: 10.1111/trf.16293. Epub 2021 Feb 8. Transfusion. 2021. PMID: 33559248 Free PMC article. Clinical Trial.
-
Screening for SARS-CoV-2 antibodies in convalescent plasma in Brazil: Preliminary lessons from a voluntary convalescent donor program.Transfusion. 2020 Dec;60(12):2938-2951. doi: 10.1111/trf.16065. Epub 2020 Sep 16. Transfusion. 2020. PMID: 32935877 Free PMC article.
-
Feasibility of a pilot program for COVID-19 convalescent plasma collection in Wuhan, China.Transfusion. 2020 Aug;60(8):1773-1777. doi: 10.1111/trf.15921. Epub 2020 Jul 31. Transfusion. 2020. PMID: 32491199 Free PMC article.
-
Ravaging SARS-CoV-2: rudimentary diagnosis and puzzling immunological responses.Curr Med Res Opin. 2021 Feb;37(2):207-217. doi: 10.1080/03007995.2020.1862532. Epub 2020 Dec 26. Curr Med Res Opin. 2021. PMID: 33306409 Free PMC article. Review.
-
The role of serum specific- SARS-CoV-2 antibody in COVID-19 patients.Int Immunopharmacol. 2021 Feb;91:107325. doi: 10.1016/j.intimp.2020.107325. Epub 2020 Dec 24. Int Immunopharmacol. 2021. PMID: 33401205 Free PMC article. Review.
Cited by
-
Digital Droplet PCR for SARS-CoV-2 Resolves Borderline Cases.Am J Clin Pathol. 2021 May 18;155(6):815-822. doi: 10.1093/ajcp/aqab041. Am J Clin Pathol. 2021. PMID: 33822853 Free PMC article.
-
Serum Level of Anti-Nucleocapsid, but Not Anti-Spike Antibody, Is Associated with Improvement of Long COVID Symptoms.Vaccines (Basel). 2022 Jan 21;10(2):165. doi: 10.3390/vaccines10020165. Vaccines (Basel). 2022. PMID: 35214624 Free PMC article.
-
Impact of serological and PCR testing requirements on the selection of COVID-19 convalescent plasma donors.Transfusion. 2021 May;61(5):1461-1470. doi: 10.1111/trf.16293. Epub 2021 Feb 8. Transfusion. 2021. PMID: 33559248 Free PMC article. Clinical Trial.
-
Pulmonary Complications and Mortality in Patients with SARS-CoV-2 Undergoing Elective and Emergent Hand Surgery.J Hand Microsurg. 2023 Jan 23;15(5):371-375. doi: 10.1055/s-0043-1760765. eCollection 2023 Dec. J Hand Microsurg. 2023. PMID: 38152673 Free PMC article.
-
Superimposed Pulmonary Tuberculosis (PTB) in a 26-Year-Old Female with No Underlying Co-Morbidities Recovering from COVID-19-Case Report.Trop Med Infect Dis. 2023 May 8;8(5):268. doi: 10.3390/tropicalmed8050268. Trop Med Infect Dis. 2023. PMID: 37235316 Free PMC article.
References
-
- Centers for Disease Control (CDC) . Recommendations for Investigational COVID‐19 Convalescent Plasma. Version updated May 1, 2020. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-in.... Accessed May 14, 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

