Novel mutations in the PLCZ1 gene associated with human low or failed fertilization
- PMID: 32840018
- PMCID: PMC7549595
- DOI: 10.1002/mgg3.1470
Novel mutations in the PLCZ1 gene associated with human low or failed fertilization
Abstract
Background: Fertilization failure (FF) is a complex reproductive disorder characterized by the failure of pronuclei formation during fertilization. In addition to some cases caused by iatrogenic problems and known genetic factors, there are still many unexplained aspects of FF. Here, we aimed to assess the clinical and genetic characteristics of two families experiencing primary infertility with FF.
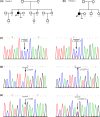
Methods: We have characterized two families from China. All of the infertile couples presented with similar clinical phenotypes, that is, partial or total fertilization failure in repeated cycles. We performed Sanger sequencing of their WEE2, TLE6, and PLCZ1 genes, and further bioinformatics and functional analyses were performed to identify the pathogenic elements of the variants.
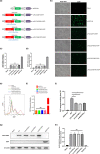
Results: We identified novel compound heterozygous mutations c.1259C>T (p.P420L) and c.1733T>C (p.M578T) in the PLCZ1 gene in a male patient of family 1 with total fertilization failure, and another novel homozygous mutation c.1727T>C (p.L576P) in the same gene in a male patient of family 2 with partial fertilization failure. These three novel mutations were absent in the control cohort and in the databases. The amino acids were conserved at their positions among six different species. All mutant amino acids were located in key domains and were predicted to impair hydrolytic activity and lead to PLCZ1 dysfunction. Further functional detection revealed that the three mutations could significantly impair the catalytic activity of PLCZ1.
Conclusions: We identified three novel mutations in PLCZ1 associated with partial and total fertilization failure and have provided new evidence about the genetic basis of FF.
Keywords: PLCZ1; fertilization failure; infertility; low fertilization; mutation.
© 2020 The Authors. Molecular Genetics & Genomic Medicine published by Wiley Periodicals LLC.
Figures

Similar articles
-
Novel phospholipase C zeta 1 mutations associated with fertilization failures after ICSI.Hum Reprod. 2019 Aug 1;34(8):1494-1504. doi: 10.1093/humrep/dez094. Hum Reprod. 2019. PMID: 31347677
-
Novel mutations in PLCZ1 cause male infertility due to fertilization failure or poor fertilization.Hum Reprod. 2020 Feb 29;35(2):472-481. doi: 10.1093/humrep/dez282. Hum Reprod. 2020. PMID: 32048714
-
Mutations in PLCZ1 induce male infertility associated with polyspermy and fertilization failure.J Assist Reprod Genet. 2023 Jan;40(1):53-64. doi: 10.1007/s10815-022-02670-2. Epub 2022 Dec 19. J Assist Reprod Genet. 2023. PMID: 36529831 Free PMC article.
-
Novel compound heterozygous mutation in WEE2 is associated with fertilization failure: case report of an infertile woman and literature review.BMC Womens Health. 2020 Nov 4;20(1):246. doi: 10.1186/s12905-020-01111-5. BMC Womens Health. 2020. PMID: 33148236 Free PMC article. Review.
-
SPERM FACTORS AND EGG ACTIVATION: Phospholipase C zeta (PLCZ1) and the clinical diagnosis of oocyte activation deficiency.Reproduction. 2022 May 23;164(1):F53-F66. doi: 10.1530/REP-21-0458. Reproduction. 2022. PMID: 35312629 Free PMC article. Review.
Cited by
-
Advances in the study of genetic factors and clinical interventions for fertilization failure.J Assist Reprod Genet. 2023 Aug;40(8):1787-1805. doi: 10.1007/s10815-023-02810-2. Epub 2023 Jun 8. J Assist Reprod Genet. 2023. PMID: 37289376 Free PMC article. Review.
-
Advances in the genetic etiology of female infertility.J Assist Reprod Genet. 2024 Dec;41(12):3261-3286. doi: 10.1007/s10815-024-03248-w. Epub 2024 Sep 25. J Assist Reprod Genet. 2024. PMID: 39320554 Review.
-
Total fertilization failure with in vitro fertilization-intracytoplasmic sperm injection related to WEE2 mutation highlights emerging importance of genetic causes of in vitro fertilization failure.F S Rep. 2022 Aug 19;3(4):355-360. doi: 10.1016/j.xfre.2022.08.007. eCollection 2022 Dec. F S Rep. 2022. PMID: 36568932 Free PMC article.
-
Phospholipase C Zeta in Human Spermatozoa: A Systematic Review on Current Development and Clinical Application.Int J Mol Sci. 2024 Jan 22;25(2):1344. doi: 10.3390/ijms25021344. Int J Mol Sci. 2024. PMID: 38279344 Free PMC article.
-
Plcz1 Deficiency Decreased Fertility in Male Mice Which Is Associated with Sperm Quality Decline and Abnormal Cytoskeleton in Epididymis.Int J Mol Sci. 2022 Dec 24;24(1):314. doi: 10.3390/ijms24010314. Int J Mol Sci. 2022. PMID: 36613757 Free PMC article.
References
-
- Dai, J. , Dai, C. , Guo, J. , Zheng, W. , Zhang, T. , Li, Y. , … Lin, G. (2020). Novel homozygous variations in PLCZ1 lead to poor or failed fertilization characterized by abnormal localization patterns of PLCζ in sperm. Clinical Genetics, 97, 347–351. - PubMed
-
- Dai, J. , Zheng, W. , Dai, C. , Guo, J. , Lu, C. , Gong, F. , … Lin, G. E. (2019). New biallelic mutations in WEE2: Expanding the spectrum of mutations that cause fertilization failure or poor fertilization. Fertility and Sterility, 111, 510–518. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

