Persistent Bacteriuria and Antibodies Recognizing Curli/eDNA Complexes From Escherichia coli Are Linked to Flares in Systemic Lupus Erythematosus
- PMID: 32840064
- PMCID: PMC7722165
- DOI: 10.1002/art.41400
Persistent Bacteriuria and Antibodies Recognizing Curli/eDNA Complexes From Escherichia coli Are Linked to Flares in Systemic Lupus Erythematosus
Abstract
Objective: Infections contribute to morbidity and mortality in systemic lupus erythematosus (SLE). Uropathogenic Escherichia coli (UPEC) are known to trigger urinary tract infections (UTIs) and form biofilms, which are multicellular communities of bacteria that are strengthened by amyloids such as curli. We previously reported that curli naturally form complexes with bacterial extracellular DNA (eDNA), and these curli/eDNA complexes induce hallmark features of lupus in mouse models. The present study was undertaken to investigate whether anti-curli/eDNA complex antibodies play a role in the pathogenesis of SLE or development of flares in SLE.
Methods: In total, 96 SLE patients who met at least 4 Systemic Lupus International Collaborating Clinics disease criteria were investigated. Anti-curli/eDNA complex antibodies in the plasma were tested for both IgG and IgA subclasses. Results were compared to that in 54 age-, sex-, and race/ethnicity-matched healthy controls. Correlations of the levels of anti-curli/eDNA antibodies with clinical parameters, lupus disease status, and frequency of bacteriuria were assessed.
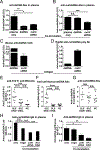
Results: Anti-curli/eDNA antibodies were detected in the plasma of SLE patients and healthy controls, and their levels correlated with the presence of asymptomatic persistent bacteriuria and occurrence of disease flares in lupus patients. Persistent bacteriuria contained curli-producing UPEC, and this was associated with an inflammatory phenotype. Finally, curli/eDNA complexes cross-reacted with lupus autoantigens, such as double-stranded DNA, in binding autoantibodies.
Conclusion: These results suggest that UTIs and persistent bacteriuria are environmental triggers of lupus and its flares. Antibodies against curli/eDNA could serve as a sign of systemic exposure to bacterial products in SLE.
© 2020, American College of Rheumatology.
Conflict of interest statement
Figures

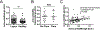

Similar articles
-
Neutrophil Extracellular Trap Mitochondrial DNA and Its Autoantibody in Systemic Lupus Erythematosus and a Proof-of-Concept Trial of Metformin.Arthritis Rheumatol. 2015 Dec;67(12):3190-200. doi: 10.1002/art.39296. Arthritis Rheumatol. 2015. PMID: 26245802 Clinical Trial.
-
Autoantibodies in Systemic Lupus Erythematosus Target Mitochondrial RNA.Front Immunol. 2019 May 10;10:1026. doi: 10.3389/fimmu.2019.01026. eCollection 2019. Front Immunol. 2019. PMID: 31134086 Free PMC article.
-
Triggers of Autoimmunity: The Role of Bacterial Infections in the Extracellular Exposure of Lupus Nuclear Autoantigens.Front Immunol. 2019 Nov 8;10:2608. doi: 10.3389/fimmu.2019.02608. eCollection 2019. Front Immunol. 2019. PMID: 31781110 Free PMC article. Review.
-
High Prevalence and Disease Correlation of Autoantibodies Against p40 Encoded by Long Interspersed Nuclear Elements in Systemic Lupus Erythematosus.Arthritis Rheumatol. 2020 Jan;72(1):89-99. doi: 10.1002/art.41054. Arthritis Rheumatol. 2020. PMID: 31342656 Free PMC article.
-
Autoantibodies in systemic lupus erythematosus.Ann Acad Med Singap. 1988 Apr;17(2):195-200. Ann Acad Med Singap. 1988. PMID: 3044259 Review.
Cited by
-
DNA at the center of mammalian innate immune recognition of bacterial biofilms.Trends Immunol. 2024 Feb;45(2):103-112. doi: 10.1016/j.it.2023.12.004. Epub 2024 Jan 27. Trends Immunol. 2024. PMID: 38281884 Free PMC article. Review.
-
Bacterial Amyloids as Hubs for Nucleic Acid Interactions: Implications and Mechanisms.Int J Mol Sci. 2025 Jul 8;26(14):6560. doi: 10.3390/ijms26146560. Int J Mol Sci. 2025. PMID: 40724810 Free PMC article. Review.
-
The role of DNA in the pathogenesis of SLE: DNA as a molecular chameleon.Ann Rheum Dis. 2024 Jun 12;83(7):830-837. doi: 10.1136/ard-2023-225266. Ann Rheum Dis. 2024. PMID: 38749573 Free PMC article. Review.
-
Amyloid-containing biofilms and autoimmunity.Curr Opin Struct Biol. 2022 Aug;75:102435. doi: 10.1016/j.sbi.2022.102435. Epub 2022 Jul 18. Curr Opin Struct Biol. 2022. PMID: 35863164 Free PMC article. Review.
-
Bacterial biofilms in the human body: prevalence and impacts on health and disease.Front Cell Infect Microbiol. 2023 Aug 30;13:1237164. doi: 10.3389/fcimb.2023.1237164. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37712058 Free PMC article. Review.
References
-
- Doaty S, Agrawal H, Bauer E, Furst DE. Infection and Lupus: Which Causes Which? Curr Rheumatol Rep. 2016;18(3):13. - PubMed
-
- Danza A, Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus. 2013;22(12):1286–94. - PubMed
-
- Pisetsky DS, Vrabie IA. Antibodies to DNA: infection or genetics? Lupus. 2009;18(13):1176–80. - PubMed
-
- Gladman DD, Hussain F, Ibanez D, Urowitz MB. The nature and outcome of infection in systemic lupus erythematosus. Lupus. 2002;11(4):234–9. - PubMed
-
- Pasoto SG, Ribeiro AC, Bonfa E. Update on infections and vaccinations in systemic lupus erythematosus and Sjogren’s syndrome. Curr Opin Rheumatol. 2014;26(5):528–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

