Atomic structure of a mitochondrial complex I intermediate from vascular plants
- PMID: 32840211
- PMCID: PMC7447434
- DOI: 10.7554/eLife.56664
Atomic structure of a mitochondrial complex I intermediate from vascular plants
Abstract
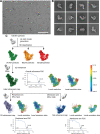
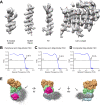
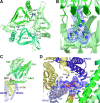
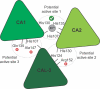
Respiration, an essential metabolic process, provides cells with chemical energy. In eukaryotes, respiration occurs via the mitochondrial electron transport chain (mETC) composed of several large membrane-protein complexes. Complex I (CI) is the main entry point for electrons into the mETC. For plants, limited availability of mitochondrial material has curbed detailed biochemical and structural studies of their mETC. Here, we present the cryoEM structure of the known CI assembly intermediate CI* from Vigna radiata at 3.9 Å resolution. CI* contains CI's NADH-binding and CoQ-binding modules, the proximal-pumping module and the plant-specific γ-carbonic-anhydrase domain (γCA). Our structure reveals significant differences in core and accessory subunits of the plant complex compared to yeast, mammals and bacteria, as well as the details of the γCA domain subunit composition and membrane anchoring. The structure sheds light on differences in CI assembly across lineages and suggests potential physiological roles for CI* beyond assembly.
Keywords: Vigna radiata; assembly; biochemistry; chemical biology; complex I; electron microscopy; mitochondria; molecular biophysics; respiration; structural biology.
Plain language summary
Respiration is the process used by all forms of life to turn organic matter from food into energy that cells can use to live and grow. The final stage of this process relies on an intricate chain of protein complexes which produce the molecule that cells use for energy. Complexes in the chain are made up of specific proteins that are carefully assembled, often into discrete modules or intermediate complexes, before coming together to form the full protein complex. Understanding how these complexes are assembled provides important insights into how respiration works. The precise three-dimensional structure of these complexes has been identified for bacteria, yeast and mammals. However, less is known about how these respiration complexes form in plants. For this reason, Maldonado et al. studied the structure of an intermediate complex that is only found in plants, called Cl*. This intermediate structure goes on to form complex I – the largest complex in the respiration chain. A technique called cryo-electron microscopy was used to obtain a structure of Cl* at a near-atomic level of detail. This structure revealed how the proteins that make up Cl* fit together, highlighting differences and similarities in how plants assemble complex I compared to bacteria, yeast and mammals. Maldonado et al. also studied the activity of Cl*, leading to the suggestion that this complex may be more than just a stepping stone towards building the full complex I and could have its own role in the cell. The structure of this complex provides new insights into the respiration mechanism of plants and could help scientists improve crop production. For instance, new compounds may be able to block respiration in pests, while leaving the crop unharmed; or genetic modifications could create plants that respire more efficiently in different environments.
© 2020, Maldonado et al.
Conflict of interest statement
MM, AP, LZ, FG, JL No competing interests declared
Figures






References
-
- Angerer H, Radermacher M, Mańkowska M, Steger M, Zwicker K, Heide H, Wittig I, Brandt U, Zickermann V. The LYR protein subunit NB4M/NDUFA6 of mitochondrial complex I anchors an acyl carrier protein and is essential for catalytic activity. PNAS. 2014;111:5207–5212. doi: 10.1073/pnas.1322438111. - DOI - PMC - PubMed

