Patient-Centered Outcomes in ARIEL3, a Phase III, Randomized, Placebo-Controlled Trial of Rucaparib Maintenance Treatment in Patients With Recurrent Ovarian Carcinoma
- PMID: 32840418
- PMCID: PMC7571791
- DOI: 10.1200/JCO.19.03107
Patient-Centered Outcomes in ARIEL3, a Phase III, Randomized, Placebo-Controlled Trial of Rucaparib Maintenance Treatment in Patients With Recurrent Ovarian Carcinoma
Abstract
Purpose: To investigate quality-adjusted progression-free survival (QA-PFS) and quality-adjusted time without symptoms or toxicity (Q-TWiST) in a post hoc exploratory analysis of the phase III ARIEL3 study of rucaparib maintenance treatment versus placebo.
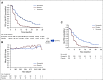
Patients and methods: Patients with platinum-sensitive, recurrent ovarian carcinoma were randomly assigned to rucaparib (600 mg twice per day) or placebo. QA-PFS was calculated as progression-free survival function × the 3-level version of the EQ-5D questionnaire (EQ-5D-3L) index score function. Q-TWiST analyses were performed defining TOX as the mean duration in which a patient experienced grade ≥ 3 treatment-emergent adverse events (TEAEs) or the mean duration in which a patient experienced grade ≥ 2 TEAEs of nausea, vomiting, fatigue, and asthenia. Q-TWiST was calculated as μTOX × TOX + TWiST, with μTOX calculated using EQ-5D-3L data.
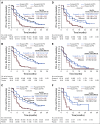
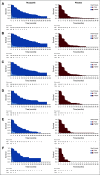
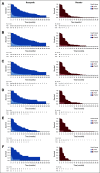
Results: The visit cutoff was Apr 15, 2017. Mean QA-PFS was significantly longer with rucaparib versus placebo in the intent-to-treat (ITT) population (375 randomly assigned to rucaparib v 189 randomly assigned to placebo; difference, 6.28 months [95% CI, 4.85 to 7.47 months]); BRCA-mutant cohort (130 rucaparib v 66 placebo; 9.37 months [95% CI, 6.65 to 11.85 months]); homologous recombination deficient (HRD) cohort (236 rucaparib v 118 placebo; 7.93 months [95% CI, 5.93 to 9.53 months]); and BRCA wild-type/loss of heterozygosity (LOH) low patient subgroup (107 rucaparib v 54 placebo; 2.71 months [95% CI, 0.31 to 4.44 months]). With TOX defined using grade ≥ 3 TEAEs, the difference in mean Q-TWiST (rucaparib v placebo) was 6.88 months (95% CI, 5.71 to 8.23 months), 9.73 months (95% CI, 7.10 to 11.94 months), 8.11 months (95% CI, 6.36 to 9.49 months), and 3.35 months (95% CI, 1.66 to 5.40 months) in the ITT population, BRCA-mutant cohort, HRD cohort, and BRCA wild-type/LOH low patient subgroup, respectively. Q-TWiST with TOX defined using select grade ≥ 2 TEAEs also consistently favored rucaparib.
Conclusion: The significant differences in QA-PFS and Q-TWiST confirm the benefit of rucaparib versus placebo in all predefined cohorts.
Figures



References
-
- Hanker LC, Loibl S, Burchardi N, et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol. 2012;23:2605–2612. - PubMed
-
- Bouberhan S, Pujade-Lauraine E, Cannistra SA. Advances in the management of platinum-sensitive relapsed ovarian cancer. J Clin Oncol. 2019;37:2424–2436. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. - PubMed
-
- Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol. 2019;30:672–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

