Single-Electron Transfer in Frustrated Lewis Pair Chemistry
- PMID: 32840947
- PMCID: PMC7756365
- DOI: 10.1002/anie.202009717
Single-Electron Transfer in Frustrated Lewis Pair Chemistry
Abstract
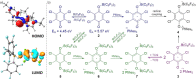
Frustrated Lewis pairs (FLPs) are well known for their ability to activate small molecules. Recent reports of radical formation within such systems indicate single-electron transfer (SET) could play an important role in their chemistry. Herein, we investigate radical formation upon reacting FLP systems with dihydrogen, triphenyltin hydride, or tetrachloro-1,4-benzoquinone (TCQ) both experimentally and computationally to determine the nature of the single-electron transfer (SET) events; that is, being direct SET to B(C6 F5 )3 or not. The reactions of H2 and Ph3 SnH with archetypal P/B FLP systems do not proceed via a radical mechanism. In contrast, reaction with TCQ proceeds via SET, which is only feasible by Lewis acid coordination to the substrate. Furthermore, SET from the Lewis base to the Lewis acid-substrate adduct may be prevalent in other reported examples of radical FLP chemistry, which provides important design principles for radical main-group chemistry.
Keywords: frustrated Lewis pairs; radicals; reactivity; single-electron transfer; substrate coordination.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





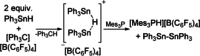


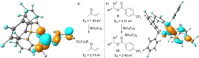
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

