A Neutral Three-Membered 2π Aromatic Disilaborirane and the Unique Conversion into a Four-Membered BSi2 N-Ring
- PMID: 32840959
- PMCID: PMC7756765
- DOI: 10.1002/anie.202009638
A Neutral Three-Membered 2π Aromatic Disilaborirane and the Unique Conversion into a Four-Membered BSi2 N-Ring
Abstract
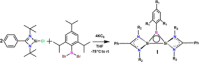
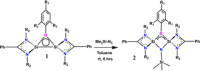
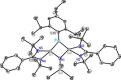
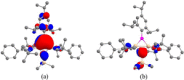
We report the design, synthesis, structure, bonding, and reaction of a neutral 2π aromatic three-membered disilaborirane. The disilaborirane is synthesized by a facile one-pot reductive dehalogenation of amidinato-silylene chloride and dibromoarylborane with potassium graphite. Despite the tetravalent arrangement of atoms around silicon, the three-membered silicon-boron-silicon ring is aromatic, as evidenced by NMR spectroscopy, nucleus independent chemical shift calculations, first-principles electronic structure studies using density functional theory (DFT) and natural bond orbital (NBO) based bonding analysis. Trimethylsilylnitrene, generated in situ, inserts in the Si-Si bond of disilaborirane to obtain a four-membered heterocycle 1-aza-2,3-disila-4-boretidine derivative. Both the heterocycles are fully characterized by X-ray crystallography.
Keywords: amidinato ligands; aromaticity; disilaborirane; molecular orbitals.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- None
-
- Minkin V. I., Glukhovtsev M. N., Simkin B. Y., Aromaticity and Antiaromaticity: Electronic and Structural Aspects, Wiley, New York, 1994;
-
- Schleyer P. v. R., Chem. Rev. 2001, 101, 1115–1118; - PubMed
-
- Balaban A. T., Schleyer P. v. R., Rzepa H. S., Chem. Rev. 2005, 105, 3436–3447; - PubMed
-
- Poater J., Duran M., Solà M., Silvi B., Chem. Rev. 2005, 105, 3911–3947; - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

