Important role of microglia in HIV-1 associated neurocognitive disorders and the molecular pathways implicated in its pathogenesis
- PMID: 32841065
- PMCID: PMC7877929
- DOI: 10.1080/07853890.2020.1814962
Important role of microglia in HIV-1 associated neurocognitive disorders and the molecular pathways implicated in its pathogenesis
Abstract
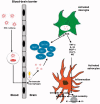
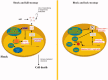
The development of effective combined anti-retroviral therapy (cART) led to a significant reduction in the death rate associated with human immunodeficiency virus type 1 (HIV-1) infection. However, recent studies indicate that considerably more than 50% of all HIV-1 infected patients develop HIV-1-associated neurocognitive disorder (HAND). Microglia are the foremost cells infected by HIV-1 in the central nervous system (CNS), and so, are also likely to contribute to the neurotoxicity observed in HAND. The activation of microglia induces the release of pro-inflammatory markers and altered secretion of cytokines, chemokines, secondary messengers, and reactive oxygen species (ROS) which activate signalling pathways that initiate neuroinflammation. In turn, ROS and inflammation also play critical roles in HAND. However, more efforts are required to understand the physiology of microglia and the processes involved in their activation in order to better understand the how HIV-1-infected microglia are involved in the development of HAND. In this review, we summarize the current state of knowledge about the involvement of oxidative stress mechanisms and role of HIV-induced ROS in the development of HAND. We also examine the academic literature regarding crucial HIV-1 pathogenicity factors implicated in neurotoxicity and inflammation in order to identify molecular pathways that could serve as potential therapeutic targets for treatment of this disease. KEY MESSAGES Neuroinflammation and excitotoxicity mechanisms are crucial in the pathogenesis of HAND. CNS infiltration by HIV-1 and immune cells through the blood brain barrier is a key process involved in the pathogenicity of HAND. Factors including calcium dysregulation and autophagy are the main challenges involved in HAND.
Keywords: Anti-retroviral therapy (ART); Chemokines (or chemotactic cytokines); HIV-1-associated neurocognitive disorders (HAND); Human immunodeficiency virus type 1 (HIV-1); Microglia; Reactive oxygen species (ROS).
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents . April 20, 2020. AIDSinfo; [cited 2020 Aug 26]. Available from: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
-
- Gougeon ML. Alarmins and central nervous system inflammation in HIV-associated neurological disorders. J Intern Med. 2017;281(5):433–447. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
