Potential role of drug metabolizing enzymes in chemotherapy-induced gastrointestinal toxicity and hepatotoxicity
- PMID: 32841068
- PMCID: PMC8059872
- DOI: 10.1080/17425255.2020.1815705
Potential role of drug metabolizing enzymes in chemotherapy-induced gastrointestinal toxicity and hepatotoxicity
Abstract
Introduction: Toxicity of chemotherapy drugs is the leading cause of poor therapeutic outcome in many cancer patients. Gastrointestinal (GI) toxicity and hepatotoxicity are among the most common side effects of current chemotherapies. Emerging studies indicate that many chemotherapy-induced toxicities are driven by drug metabolism, but very few reviews summarize the role of drug metabolism in chemotherapy-induced GI toxicity and hepatotoxicity. In this review, we highlighted the importance of drug metabolizing enzymes (DMEs) in chemotherapy toxicity.
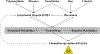
Areas covered: Our review demonstrated that altered activity of DMEs play important role in chemotherapy-induced GI toxicity and hepatotoxicity. Besides direct changes in catalytic activities, the transcription of DMEs is also affected by inflammation, cell-signaling pathways, and/or by drugs in cancer patients due to the disease etiology.
Expert opinion: More studies should focus on how DMEs are altered during chemotherapy treatment, and how such changes affect the metabolism of chemotherapy drug itself. This mutual interaction between chemotherapies and DMEs can lead to excessive exposure of parent drug or toxic metabolites which ultimately cause GI adverse effect.
Keywords: Drug metabolizing enzymes; chemotherapy; gastrointestinal toxicity; hepatotoxicity; reactive metabolites.
Figures


References
-
- Kehrer DFS, Sparreboom A, Verweij J, et al. Modulation of irinotecan-induced diarrhea by cotreatment with neomycin in cancer patients. Clin Cancer Res. 2001;7(5):1136–41. - PubMed
-
- Paranjpe R, Basatneh D, Tao G, et al. Neratinib in HER2-Positive Breast Cancer Patients. Ann Pharmacother. 2019;53(6):612–620. - PubMed
-
- Takimoto CH, Lu ZH, Zhang R, et al. Severe neurotoxicity following 5-fluorouracil-based chemotherapy in a patient with dihydropyrimidine dehydrogenase deficiency. Clin Cancer Res. 1996;2(3):477–481. - PubMed
-
- Innocenti F, Iyer L, Ramírez J, et al. Epirubicin glucuronidation is catalyzed by human UDP-glucuronosyltransferase 2B7. Drug Metab Dispos. 2001;29(5):686–692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
