Human angiotensin-converting enzyme 2 transgenic mice infected with SARS-CoV-2 develop severe and fatal respiratory disease
- PMID: 32841215
- PMCID: PMC7566707
- DOI: 10.1172/jci.insight.142032
Human angiotensin-converting enzyme 2 transgenic mice infected with SARS-CoV-2 develop severe and fatal respiratory disease
Abstract
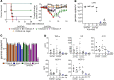
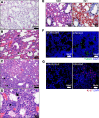
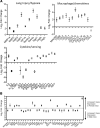
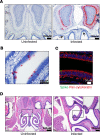
The emergence of SARS-CoV-2 has created an international health crisis, and small animal models mirroring SARS-CoV-2 human disease are essential for medical countermeasure (MCM) development. Mice are refractory to SARS-CoV-2 infection owing to low-affinity binding to the murine angiotensin-converting enzyme 2 (ACE2) protein. Here, we evaluated the pathogenesis of SARS-CoV-2 in male and female mice expressing the human ACE2 gene under the control of the keratin 18 promoter (K18). In contrast to nontransgenic mice, intranasal exposure of K18-hACE2 animals to 2 different doses of SARS-CoV-2 resulted in acute disease, including weight loss, lung injury, brain infection, and lethality. Vasculitis was the most prominent finding in the lungs of infected mice. Transcriptomic analysis from lungs of infected animals showed increases in transcripts involved in lung injury and inflammatory cytokines. In the low-dose challenge groups, there was a survival advantage in the female mice, with 60% surviving infection, whereas all male mice succumbed to disease. Male mice that succumbed to disease had higher levels of inflammatory transcripts compared with female mice. To our knowledge, this is the first highly lethal murine infection model for SARS-CoV-2 and should be valuable for the study of SARS-CoV-2 pathogenesis and for the assessment of MCMs.
Keywords: COVID-19; Infectious disease; Molecular pathology; Mouse models.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

