Radical-Based Synthesis and Modification of Amino Acids
- PMID: 32841470
- PMCID: PMC7820943
- DOI: 10.1002/anie.202010157
Radical-Based Synthesis and Modification of Amino Acids
Abstract
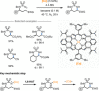
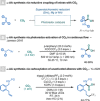
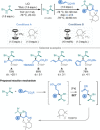
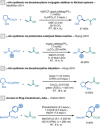
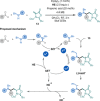
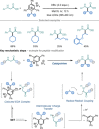
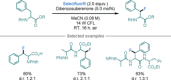
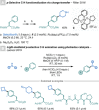
Amino acids (AAs) are key structural motifs with widespread applications in organic synthesis, biochemistry, and material sciences. Recently, with the development of milder and more versatile radical-based procedures, the use of strategies relying on radical chemistry for the synthesis and modification of AAs has gained increased attention, as they allow rapid access to libraries of novel unnatural AAs containing a wide range of structural motifs. In this Minireview, we provide a broad overview of the advancements made in this field during the last decade, focusing on methods for the de novo synthesis of α-, β-, and γ-AAs, as well as for the selective derivatisation of canonical and non-canonical α-AAs.
Keywords: amino acids; peptides; photochemistry; radical reactions; synthetic methods.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
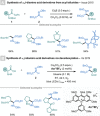

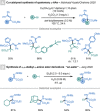
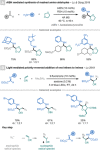
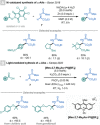


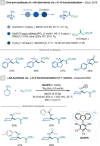
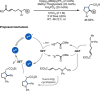


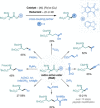
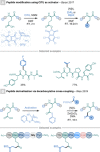






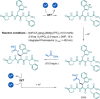

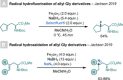
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

