VEGF-C protects the integrity of the bone marrow perivascular niche in mice
- PMID: 32842144
- PMCID: PMC7568034
- DOI: 10.1182/blood.2020005699
VEGF-C protects the integrity of the bone marrow perivascular niche in mice
Abstract
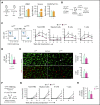
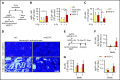
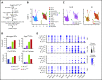
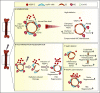
Hematopoietic stem cells (HSCs) reside in the bone marrow (BM) stem cell niche, which provides a vital source of HSC regulatory signals. Radiation and chemotherapy disrupt the HSC niche, including its sinusoidal vessels and perivascular cells, contributing to delayed hematopoietic recovery. Thus, identification of factors that can protect the HSC niche during an injury could offer a significant therapeutic opportunity to improve hematopoietic regeneration. In this study, we identified a critical function for vascular endothelial growth factor-C (VEGF-C), that of maintaining the integrity of the BM perivascular niche and improving BM niche recovery after irradiation-induced injury. Both global and conditional deletion of Vegfc in endothelial or leptin receptor-positive (LepR+) cells led to a disruption of the BM perivascular niche. Furthermore, deletion of Vegfc from the microenvironment delayed hematopoietic recovery after transplantation by decreasing endothelial proliferation and LepR+ cell regeneration. Exogenous administration of VEGF-C via an adenoassociated viral vector improved hematopoietic recovery after irradiation by accelerating endothelial and LepR+ cell regeneration and by increasing the expression of hematopoietic regenerative factors. Our results suggest that preservation of the integrity of the perivascular niche via VEGF-C signaling could be exploited therapeutically to enhance hematopoietic regeneration.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
Similar articles
-
Hematopoietic stem and progenitor cells regulate the regeneration of their niche by secreting Angiopoietin-1.Elife. 2015 Mar 30;4:e05521. doi: 10.7554/eLife.05521. Elife. 2015. PMID: 25821987 Free PMC article.
-
Piezo1-mediated mechanosensation in bone marrow macrophages promotes vascular niche regeneration after irradiation injury.Theranostics. 2022 Jan 16;12(4):1621-1638. doi: 10.7150/thno.64963. eCollection 2022. Theranostics. 2022. PMID: 35198061 Free PMC article.
-
Microbiota-derived lactate promotes hematopoiesis and erythropoiesis by inducing stem cell factor production from leptin receptor+ niche cells.Exp Mol Med. 2021 Sep;53(9):1319-1331. doi: 10.1038/s12276-021-00667-y. Epub 2021 Sep 9. Exp Mol Med. 2021. PMID: 34497346 Free PMC article.
-
From Marrow to Bone and Fat: Exploring the Multifaceted Roles of Leptin Receptor Positive Bone Marrow Mesenchymal Stromal Cells.Cells. 2024 May 24;13(11):910. doi: 10.3390/cells13110910. Cells. 2024. PMID: 38891042 Free PMC article. Review.
-
Complexity of bone marrow hematopoietic stem cell niche.Int J Hematol. 2017 Jul;106(1):45-54. doi: 10.1007/s12185-017-2262-9. Epub 2017 May 22. Int J Hematol. 2017. PMID: 28534115 Free PMC article. Review.
Cited by
-
Proteolytic Cleavages in the VEGF Family: Generating Diversity among Angiogenic VEGFs, Essential for the Activation of Lymphangiogenic VEGFs.Biology (Basel). 2021 Feb 23;10(2):167. doi: 10.3390/biology10020167. Biology (Basel). 2021. PMID: 33672235 Free PMC article. Review.
-
Far from Health: The Bone Marrow Microenvironment in AML, A Leukemia Supportive Shelter.Children (Basel). 2021 May 8;8(5):371. doi: 10.3390/children8050371. Children (Basel). 2021. PMID: 34066861 Free PMC article. Review.
-
Neuropilin 1 regulates bone marrow vascular regeneration and hematopoietic reconstitution.Nat Commun. 2021 Nov 30;12(1):6990. doi: 10.1038/s41467-021-27263-y. Nat Commun. 2021. PMID: 34848712 Free PMC article.
-
Lotus seedpod-inspired internal vascularized 3D printed scaffold for bone tissue repair.Bioact Mater. 2020 Nov 27;6(6):1639-1652. doi: 10.1016/j.bioactmat.2020.11.019. eCollection 2021 Jun. Bioact Mater. 2020. PMID: 33313444 Free PMC article.
-
From the niche to malignant hematopoiesis and back: reciprocal interactions between leukemia and the bone marrow microenvironment.JBMR Plus. 2021 Jun 3;5(10):e10516. doi: 10.1002/jbm4.10516. eCollection 2021 Oct. JBMR Plus. 2021. PMID: 34693187 Free PMC article.
References
-
- Ramasamy SK, Kusumbe AP, Itkin T, Gur-Cohen S, Lapidot T, Adams RH. Regulation of Hematopoiesis and Osteogenesis by Blood Vessel-Derived Signals. Annu Rev Cell Dev Biol. 2016;32(1):649-675. - PubMed
-
- Bernad A, Kopf M, Kulbacki R, Weich N, Koehler G, Gutierrez-Ramos JCH. Interleukin-6 is required in vivo for the regulation of stem cells and committed progenitors of the hematopoietic system. Immunity. 1994;1(9):725-731. - PubMed
-
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977-988. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

