N-glycosylation in the Pre-Membrane Protein Is Essential for the Zika Virus Life Cycle
- PMID: 32842538
- PMCID: PMC7552079
- DOI: 10.3390/v12090925
N-glycosylation in the Pre-Membrane Protein Is Essential for the Zika Virus Life Cycle
Abstract
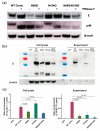
Asparagine (N)-linked protein glycosylation plays an important role in protein synthesis and modification. Two Zika virus (ZIKV) structural proteins, the pre-membrane (prM) and envelope (E) protein are N-glycosylated. The prM protein of all ZIKV strains contains a single N-linked glycosylation site, while not all strains contain an N-linked site in the E protein. Our aim was to examine the impact of prM and E N-linked glycosylation on ZIKV infectivity and cell trafficking. Using a ZIKV infectious clone, we found that when the N-glycan sites were removed, the prM- and the prM/E-double mutants did not produce an infectious virus in the supernatant. Further, by using ZIKV prME constructs, we found that N-glycosylation was necessary for effective secretion of ZIKV virions. The absence of the N-glycan on prM or E caused protein aggregation in the rough endoplasmatic reticulum (ER) compartment. The aggregation was more pronounced for the prM-mutation, and the mutant virus lost the ER-Golgi intermediate compartment (ERGIC) localization. In addition, lack of the N-glycan on prM induced nuclear translocation of CCAAT-enhancer-binding protein homologous protein (CHOP), an ER stress marker. To conclude, we show that the prM N-glycan is essential for the ZIKV infectious cycle, and plays an important role in viral protein trafficking, protein folding, and virion assembly.
Keywords: N-glycosylation; Zika virus; envelope; pre-membrane; virus life cycle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

