Elucidating the Inhibitory Effect of Resveratrol and Its Structural Analogs on Selected Nucleotide-Related Enzymes
- PMID: 32842666
- PMCID: PMC7563984
- DOI: 10.3390/biom10091223
Elucidating the Inhibitory Effect of Resveratrol and Its Structural Analogs on Selected Nucleotide-Related Enzymes
Abstract
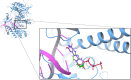
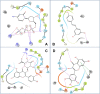
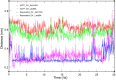
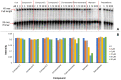
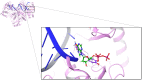
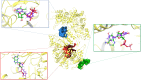
Resveratrol, the most widely studied natural phytochemical, has been shown to interact with different target proteins. Previous studies show that resveratrol binds and inhibits DNA polymerases and some other enzymes; however, the binding and functioning mechanisms remain unknown. The elucidated knowledge of inhibitory mechanisms of resveratrol will assist us in new drug discovery. We utilized molecular docking and molecular dynamics (MD) simulation to reveal how resveratrol and structurally similar compounds bind to various nucleotide-dependent enzymes, specifically, DNA polymerases, HIV-1 reverse transcriptase, and ribonucleotide reductase. The results show that resveratrol and its analogs exert their inhibitory effects by competing with the substrate dNTPs in these enzymes and blocking elongation of chain polymerization. In addition, the results imply that resveratrol binds to a variety of other ATP-/NTP-binding proteins.
Keywords: inhibition mechanism; molecular docking; molecular dynamics simulation; nucleotide-related enzymes; resveratrol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hsieh T.-c., editor. Resveratrol: State-of-the-Art Science and Health Applications-Actionable Targets and Mechanisms Of Resveratrol. World Scientific; Singapore: 2018.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

