Analysis of Early Cone Dysfunction in an In Vivo Model of Rod-Cone Dystrophy
- PMID: 32842706
- PMCID: PMC7503557
- DOI: 10.3390/ijms21176055
Analysis of Early Cone Dysfunction in an In Vivo Model of Rod-Cone Dystrophy
Abstract
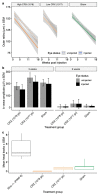
Retinitis pigmentosa (RP) is a generic term for a group of genetic diseases characterized by loss of rod and cone photoreceptor cells. Although the genetic causes of RP frequently only affect the rod photoreceptor cells, cone photoreceptors become stressed in the absence of rods and undergo a secondary degeneration. Changes in the gene expression profile of cone photoreceptor cells are likely to occur prior to observable physiological changes. To this end, we sought to achieve greater understanding of the changes in cone photoreceptor cells early in the degeneration process of the Rho-/- mouse model. To account for gene expression changes attributed to loss of cone photoreceptor cells, we normalized PCR in the remaining number of cones to a cone cell reporter (OPN1-GFP). Gene expression profiles of key components involved in the cone phototransduction cascade were correlated with tests of retinal cone function prior to cell loss. A significant downregulation of the photoreceptor transcription factor Crx was observed, which preceded a significant downregulation in cone opsin transcripts that coincided with declining cone function. Our data add to the growing understanding of molecular changes that occur prior to cone dysfunction in a model of rod-cone dystrophy. It is of interest that gene supplementation of CRX by adeno-associated viral vector delivery prior to cone cell loss did not prevent cone photoreceptor degeneration in this mouse model.
Keywords: cone photoreceptors; gene therapy; retina; retinitis pigmentosa; rod-cone dystrophy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

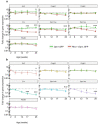
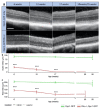
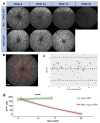
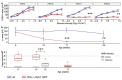

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

