Rapid establishment of a COVID-19 perinatal biorepository: early lessons from the first 100 women enrolled
- PMID: 32842979
- PMCID: PMC7447612
- DOI: 10.1186/s12874-020-01102-y
Rapid establishment of a COVID-19 perinatal biorepository: early lessons from the first 100 women enrolled
Abstract
Background: Collection of biospecimens is a critical first step to understanding the impact of COVID-19 on pregnant women and newborns - vulnerable populations that are challenging to enroll and at risk of exclusion from research. We describe the establishment of a COVID-19 perinatal biorepository, the unique challenges imposed by the COVID-19 pandemic, and strategies used to overcome them.
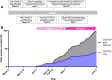
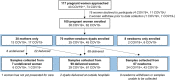
Methods: A transdisciplinary approach was developed to maximize the enrollment of pregnant women and their newborns into a COVID-19 prospective cohort and tissue biorepository, established on March 19, 2020 at Massachusetts General Hospital (MGH). The first SARS-CoV-2 positive pregnant woman was enrolled on April 2, and enrollment was expanded to SARS-CoV-2 negative controls on April 20. A unified enrollment strategy with a single consent process for pregnant women and newborns was implemented on May 4. SARS-CoV-2 status was determined by viral detection on RT-PCR of a nasopharyngeal swab. Wide-ranging and pregnancy-specific samples were collected from maternal participants during pregnancy and postpartum. Newborn samples were collected during the initial hospitalization.
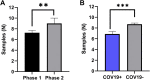
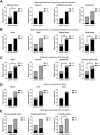
Results: Between April 2 and June 9, 100 women and 78 newborns were enrolled in the MGH COVID-19 biorepository. The rate of dyad enrollment and number of samples collected per woman significantly increased after changes to enrollment strategy (from 5 to over 8 dyads/week, P < 0.0001, and from 7 to 9 samples, P < 0.01). The number of samples collected per woman was higher in SARS-CoV-2 negative than positive women (9 vs 7 samples, P = 0.0007). The highest sample yield was for placenta (96%), umbilical cord blood (93%), urine (99%), and maternal blood (91%). The lowest-yield sample types were maternal stool (30%) and breastmilk (22%). Of the 61 delivered women who also enrolled their newborns, fewer women agreed to neonatal blood compared to cord blood (39 vs 58, P < 0.0001).
Conclusions: Establishing a COVID-19 perinatal biorepository required patient advocacy, transdisciplinary collaboration and creative solutions to unique challenges. This biorepository is unique in its comprehensive sample collection and the inclusion of a control population. It serves as an important resource for research into the impact of COVID-19 on pregnant women and newborns and provides lessons for future biorepository efforts.
Keywords: Biobank; COVID-19; Immune; Neonatology; Newborn; Obstetrics; Pandemic; Pregnancy; Repository; SARS-CoV-2; Vertical transmission.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University [https://coronavirus.jhu.edu/map.html].
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

