Differential contribution of Anopheles coustani and Anopheles arabiensis to the transmission of Plasmodium falciparum and Plasmodium vivax in two neighbouring villages of Madagascar
- PMID: 32843082
- PMCID: PMC7447585
- DOI: 10.1186/s13071-020-04282-0
Differential contribution of Anopheles coustani and Anopheles arabiensis to the transmission of Plasmodium falciparum and Plasmodium vivax in two neighbouring villages of Madagascar
Abstract
Background: Malaria is still a heavy public health concern in Madagascar. Few studies combining parasitology and entomology have been conducted despite the need for accurate information to design effective vector control measures. In a Malagasy region of moderate to intense transmission of both Plasmodium falciparum and P. vivax, parasitology and entomology have been combined to survey malaria transmission in two nearby villages.
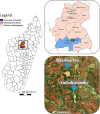
Methods: Community-based surveys were conducted in the villages of Ambohitromby and Miarinarivo at three time points (T1, T2 and T3) during a single malaria transmission season. Human malaria prevalence was determined by rapid diagnostic tests (RDTs), microscopy and real-time PCR. Mosquitoes were collected by human landing catches and pyrethrum spray catches and the presence of Plasmodium sporozoites was assessed by TaqMan assay.
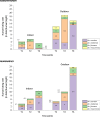
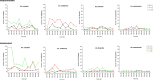
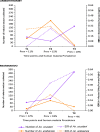
Results: Malaria prevalence was not significantly different between villages, with an average of 8.0% by RDT, 4.8% by microscopy and 11.9% by PCR. This was mainly due to P. falciparum and to a lesser extent to P. vivax. However, there was a significantly higher prevalence rate as determined by PCR at T2 ([Formula: see text] = 7.46, P = 0.025). Likewise, mosquitoes were significantly more abundant at T2 ([Formula: see text] = 64.8, P < 0.001), especially in Ambohitromby. At T1 and T3 mosquito abundance was higher in Miarinarivo than in Ambohitromby ([Formula: see text] = 14.92, P < 0.001). Of 1550 Anopheles mosquitoes tested, 28 (1.8%) were found carrying Plasmodium sporozoites. The entomological inoculation rate revealed that Anopheles coustani played a major contribution in malaria transmission in Miarinarivo, being responsible of 61.2 infective bites per human (ib/h) during the whole six months of the survey, whereas, it was An. arabiensis, with 36 ib/h, that played that role in Ambohitromby.
Conclusions: Despite a similar malaria prevalence in two nearby villages, the entomological survey showed a different contribution of An. coustani and An. arabiensis to malaria transmission in each village. Importantly, the suspected secondary malaria vector An. coustani, was found playing the major role in malaria transmission in one village. This highlights the importance of combining parasitology and entomology surveys for better targeting local malaria vectors. Such study should contribute to the malaria pre-elimination goal established under the 2018-2022 National Malaria Strategic Plan.
Keywords: Andriba; Anopheles arabiensis; Anopheles coustani; Madagascar; Plasmodium falciparum; Plasmodium vivax; Vector biology dynamics.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- World Health Organization. World malaria report 2019. Geneva: World Health Organization; 2019. https://www.who.int/publications-detail/world-malaria-report-2019. Accessed 9 Mar 2020.
-
- Mouchet J, Blanchy S, Rakotonjanabelo A, Ranaivoson G, Rajaonarivelo E, Laventure S, et al. Epidemiological stratification of malaria in Madagascar. Arch Inst Pasteur Madagascar. 1993;60:50–59. - PubMed
-
- Howes RE, Mioramalala SA, Ramiranirina B, Franchard T, Rakotorahalahy AJ, Bisanzio D, et al. Contemporary epidemiological overview of malaria in Madagascar: operational utility of reported routine case data for malaria control planning. Malar J. 2016;15:502. doi: 10.1186/s12936-016-1556-3. - DOI - PMC - PubMed
-
- Kang SY, Battle KE, Gibson HS, Ratsimbasoa A, Randrianarivelojosia M, Ramboarina S, et al. Spatio-temporal mapping of Madagascar’s Malaria Indicator Survey results to assess Plasmodium falciparum endemicity trends between 2011 and 2016. BMC Med. 2018;16:71. doi: 10.1186/s12916-018-1060-4. - DOI - PMC - PubMed
MeSH terms
Grants and funding
- DI/EC/MAM/N°479/14/Institut Pasteur International Network (FR)
- IPIN/G4 GROUP-02/Institut Pasteur International Network (FR)
- ANR-10-LABX-62-IBEID/Agence Nationale de la Recherche (FR)
- IPM/IPal-VivaxDuffy Internal Grant/Institut Pasteur de Madagascar (MG)
- 007/IPM/DIR/PR/16/Institut Pasteur de Madagascar (MG)
LinkOut - more resources
Full Text Sources

