Efficacy and Safety of Expanded Hemodialysis with the Theranova 400 Dialyzer: A Randomized Controlled Trial
- PMID: 32843372
- PMCID: PMC7480550
- DOI: 10.2215/CJN.01210120
Efficacy and Safety of Expanded Hemodialysis with the Theranova 400 Dialyzer: A Randomized Controlled Trial
Abstract
Background and objectives: Expanded hemodialysis therapy enabled by medium cut-off membranes may promote greater clearance of larger middle molecules that comprise putative uremic solutes than conventional high-flux dialysis. This randomized trial evaluated the efficacy and safety of hemodialysis treatment with a medium cut-off dialyzer.
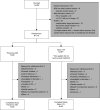
Design, setting, participants, & measurements: Clinically stable patients on maintenance hemodialysis were randomized to receive dialysis with either a medium cut-off dialyzer (Theranova 400) or a high-flux dialyzer (Elisio-17H) over 24 weeks of treatment. The primary safety end point was the predialysis serum albumin level after 24 weeks of treatment. The primary efficacy end point was the reduction ratio of free λ light chains at 24 weeks of treatment.
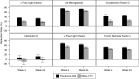
Results: Among 172 patients on maintenance hemodialysis, mean age was 59±13 years, 61% were men, 40% were Black, and mean dialysis vintage was 5±4 years. Of the 86 patients randomized to each dialyzer, 65 completed the trial in each group. The reduction ratio for the removal of free λ light chains was significantly higher in the Theranova 400 group compared with the Elisio-17H group after 4 weeks (39% versus 20%) and 24 weeks (33% versus 17%; both P<0.001). Among secondary end points, the Theranova 400 group demonstrated significantly larger reduction ratios at 4 and 24 weeks for complement factor D, free κ light chains, TNFα, and β2-microglobulin (P<0.001 for all), but not for IL-6. Predialysis serum albumin levels were similar between groups after 24 weeks (4 g/dl with the Theranova 400 and 4.1 g/dl with the Elisio-17H), consistent with noninferiority of the Theranova 400 dialyzer in maintaining predialysis serum albumin levels after 24 weeks of treatment.
Conclusions: Hemodialysis therapy with the Theranova 400 dialyzer provides superior removal of larger middle molecules, as exemplified by free λ light chains, compared with a similar size high-flux dialyzer, while maintaining serum albumin level.
Clinical trial registry name and registration number: A Multi-Center, Prospective, Randomized, Controlled, Open-Label, Parallel Study to Evaluate the Safety and Efficacy of the Theranova 400 Dialyzer in End Stage Renal Disease (ESRD) Patients, NCT03257410.
Keywords: ESKD; chronic dialysis; chronic inflammation; dialysis; end-stage kidney disease; hemodialysis; maintenance dialysis; randomized controlled trials; renal dialysis.
Copyright © 2020 by the American Society of Nephrology.
Figures

References
-
- Meyer TW, Hostetter TH: Uremia. N Engl J Med 357: 1316–1325, 2007. - PubMed
-
- Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W; European Uremic Toxin Work Group (EUTox) : Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int 63: 1934–1943, 2003. - PubMed
-
- Hutchison CA, Wolley M: The rationale for expanded hemodialysis therapy (HDx). Contrib Nephrol 191: 142–152, 2017. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

