Establishment of Proximity-Dependent Biotinylation Approaches in Different Plant Model Systems
- PMID: 32843435
- PMCID: PMC7610282
- DOI: 10.1105/tpc.20.00235
Establishment of Proximity-Dependent Biotinylation Approaches in Different Plant Model Systems
Erratum in
-
Correction to: Establishment of Proximity-Dependent Biotinylation Approaches in Different Plant Model Systems.Plant Cell. 2022 Jul 4;34(7):2806. doi: 10.1093/plcell/koac101. Plant Cell. 2022. PMID: 35357494 Free PMC article. No abstract available.
Abstract
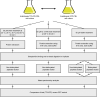
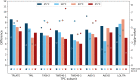
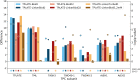
Proximity labeling is a powerful approach for detecting protein-protein interactions. Most proximity labeling techniques use a promiscuous biotin ligase or a peroxidase fused to a protein of interest, enabling the covalent biotin labeling of proteins and subsequent capture and identification of interacting and neighboring proteins without the need for the protein complex to remain intact. To date, only a few studies have reported on the use of proximity labeling in plants. Here, we present the results of a systematic study applying a variety of biotin-based proximity labeling approaches in several plant systems using various conditions and bait proteins. We show that TurboID is the most promiscuous variant in several plant model systems and establish protocols that combine mass spectrometry-based analysis with harsh extraction and washing conditions. We demonstrate the applicability of TurboID in capturing membrane-associated protein interactomes using Lotus japonicus symbiotically active receptor kinases as a test case. We further benchmark the efficiency of various promiscuous biotin ligases in comparison with one-step affinity purification approaches. We identified both known and novel interactors of the endocytic TPLATE complex. We furthermore present a straightforward strategy to identify both nonbiotinylated and biotinylated peptides in a single experimental setup. Finally, we provide initial evidence that our approach has the potential to suggest structural information of protein complexes.
© 2020 American Society of Plant Biologists. All rights reserved.
Figures






References
-
- Alban C., Job D., Douce R.(2000). Biotin metabolism in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51: 17–47. - PubMed
-
- Antolín-Llovera M., Ried M.K., Parniske M.(2014). Cleavage of the SYMBIOSIS RECEPTOR-LIKE KINASE ectodomain promotes complex formation with Nod factor receptor 5. Curr. Biol. 24: 422–427. - PubMed
-
- Batsios P., Meyer I., Gräf R.(2016). Proximity-dependent biotin identification (BioID) in Dictyostelium amoebae. Methods Enzymol. 569: 23–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

