A post-invasion role for Chlamydia type III effector TarP in modulating the dynamics and organization of host cell focal adhesions
- PMID: 32843479
- PMCID: PMC7586217
- DOI: 10.1074/jbc.RA120.015219
A post-invasion role for Chlamydia type III effector TarP in modulating the dynamics and organization of host cell focal adhesions
Abstract
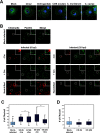
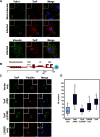
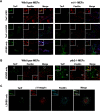
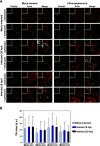
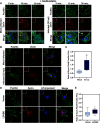
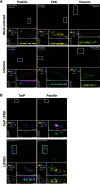
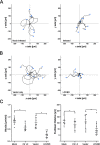
The human pathogen Chlamydia trachomatis targets epithelial cells lining the genital mucosa. We observed that infection of various cell types, including fibroblasts and epithelial cells resulted in the formation of unusually stable and mature focal adhesions that resisted disassembly induced by the myosin II inhibitor, blebbistatin. Superresolution microscopy revealed in infected cells the vertical displacement of paxillin and focal adhesion kinase from the signaling layer of focal adhesions, whereas vinculin remained in its normal position within the force transduction layer. The candidate type III effector TarP, which localized to focal adhesions during infection and when expressed ectopically, was sufficient to mimic both the reorganization and blebbistatin-resistant phenotypes. These effects of TarP, including its localization to focal adhesions, required a post-invasion interaction with the host protein vinculin through a specific domain at the C terminus of TarP. This interaction is repurposed from an actin-recruiting and -remodeling complex to one that mediates nanoarchitectural and dynamic changes of focal adhesions. The consequence of Chlamydia-stabilized focal adhesions was restricted cell motility and enhanced attachment to the extracellular matrix. Thus, via a novel mechanism, Chlamydia inserts TarP within focal adhesions to alter their organization and stability.
Keywords: Chlamydia; Chlamydia trachomatis; bacterial pathogenesis; cell adhesion; cell motility; focal adhesion; focal adhesions; pathogenesis.
© 2020 Pedrosa et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

