YAP Activation in Renal Proximal Tubule Cells Drives Diabetic Renal Interstitial Fibrogenesis
- PMID: 32843569
- PMCID: PMC7576565
- DOI: 10.2337/db20-0579
YAP Activation in Renal Proximal Tubule Cells Drives Diabetic Renal Interstitial Fibrogenesis
Abstract
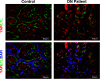
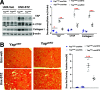
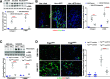
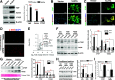
An increasing number of studies suggest that the renal proximal tubule is a site of injury in diabetic nephropathy (DN), and progressive renal tubulointerstitial fibrosis is an important mediator of progressive kidney dysfunction in DN. In this study, we observed increased expression and activation of YAP (yes-associated protein) in renal proximal tubule epithelial cells (RPTC) in patients with diabetes and in mouse kidneys. Inducible deletion of Yap specifically in RPTC or administration of the YAP inhibitor verteporfin significantly attenuated diabetic tubulointerstitial fibrosis. EGFR-dependent activation of RhoA/Rock and PI3K-Akt signals and their reciprocal interaction were upstream of proximal tubule YAP activation in diabetic kidneys. Production and release of CTGF in culture medium were significantly augmented in human embryonic kidney (HEK)-293 cells transfected with a constitutively active YAP mutant, and the conditioned medium collected from these cells activated and transduced fibroblasts into myofibroblasts. This study demonstrates that proximal tubule YAP-dependent paracrine mechanisms play an important role in diabetic interstitial fibrogenesis; therefore, targeting Hippo signaling may be a therapeutic strategy to prevent the development and progression of diabetic interstitial fibrogenesis.
© 2020 by the American Diabetes Association.
Figures



References
-
- Atkins RC, Zimmet P. Diabetic kidney disease: act now or pay later. Kidney Int 2010;77:375–377 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

