TRPV4 activates the Cdc42/N-wasp pathway to promote glioblastoma invasion by altering cellular protrusions
- PMID: 32843668
- PMCID: PMC7447819
- DOI: 10.1038/s41598-020-70822-4
TRPV4 activates the Cdc42/N-wasp pathway to promote glioblastoma invasion by altering cellular protrusions
Abstract
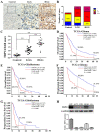
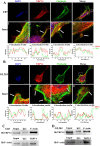
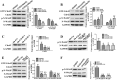
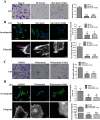
The invasion ability of glioblastoma (GBM) causes tumor cells to infiltrate the surrounding brain parenchyma and leads to poor outcomes. Transient receptor potential vanilloid 4 (TRPV4) exhibits a remarkable role in cancer cell motility, but the contribution of TRPV4 to glioblastoma metastasis is not fully understood. Here, we reported that TRPV4 expression was significantly elevated in malignant glioma compared to normal brain and low-grade glioma, and TRPV4 expression was negatively correlated with the prognosis of glioma patients. Functionally, stimulation of TRPV4 promoted glioblastoma cell migration and invasion, and repression of TRPV4 hindered the migration and invasion of glioblastoma cells in vitro. Molecularly, TRPV4 strongly colocalized and interacted with skeletal protein-F-actin at cellular protrusions, and TRPV4 regulated the formation of invadopodia and filopodia in glioblastoma cells. Furthermore, the Cdc42/N-wasp axis mediated the effect of TRPV4-regulated cellular protrusions and invasion. Foremost, TRPV4 inhibitor treatment or downregulation of TRPV4 significantly reduced the invasion-growth of subcutaneously and intracranially transplanted glioblastoma in mice. In conclusion, the TRPV4/Cdc42/wasp signaling axis regulates cellular protrusion formation in glioblastoma cells and influences the invasion-growth phenotype of glioblastoma in vivo. TRPV4 may serve as a prognostic factor and specific therapeutic target for GBM patients.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

