Sensory satellite glial Gq-GPCR activation alleviates inflammatory pain via peripheral adenosine 1 receptor activation
- PMID: 32843670
- PMCID: PMC7447794
- DOI: 10.1038/s41598-020-71073-z
Sensory satellite glial Gq-GPCR activation alleviates inflammatory pain via peripheral adenosine 1 receptor activation
Abstract
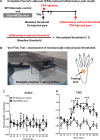
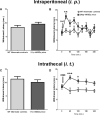
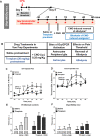
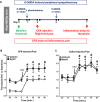
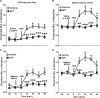
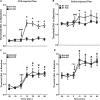
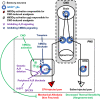
Glial fibrillary acidic protein expressing (GFAP+) glia modulate nociceptive neuronal activity in both the peripheral nervous system (PNS) and the central nervous system (CNS). Resident GFAP+ glia in dorsal root ganglia (DRG) known as satellite glial cells (SGCs) potentiate neuronal activity by releasing pro-inflammatory cytokines and neuroactive compounds. In this study, we tested the hypothesis that SGC Gq-coupled receptor (Gq-GPCR) signaling modulates pain sensitivity in vivo using Gfap-hM3Dq mice. Complete Freund's adjuvant (CFA) was used to induce inflammatory pain, and mechanical sensitivity and thermal sensitivity were used to assess the neuromodulatory effect of glial Gq-GPCR activation in awake mice. Pharmacogenetic activation of Gq-GPCR signaling in sensory SGCs decreased heat-induced nociceptive responses and reversed inflammation-induced mechanical allodynia via peripheral adenosine A1 receptor activation. These data reveal a previously unexplored role of sensory SGCs in decreasing afferent excitability. The identified molecular mechanism underlying the analgesic role of SGCs offers new approaches for reversing peripheral nociceptive sensitization.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Ganglionic GFAP + glial Gq-GPCR signaling enhances heart functions in vivo.JCI Insight. 2017 Jan 26;2(2):e90565. doi: 10.1172/jci.insight.90565. JCI Insight. 2017. PMID: 28138563 Free PMC article.
-
Satellite glial cell-secreted exosomes after in-vitro oxaliplatin treatment presents a pro-nociceptive effect for dorsal root ganglion neurons and induce mechanical hypersensitivity in naïve mice.Mol Cell Neurosci. 2023 Sep;126:103881. doi: 10.1016/j.mcn.2023.103881. Epub 2023 Jul 18. Mol Cell Neurosci. 2023. PMID: 37467904
-
Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior.Mol Pain. 2010 Apr 15;6:21. doi: 10.1186/1744-8069-6-21. Mol Pain. 2010. PMID: 20398327 Free PMC article.
-
Recent advances in basic research on the trigeminal ganglion.J Physiol Sci. 2016 Sep;66(5):381-6. doi: 10.1007/s12576-016-0448-1. Epub 2016 Mar 29. J Physiol Sci. 2016. PMID: 27023716 Free PMC article. Review.
-
Satellite Glial Cells in Human Disease.Cells. 2024 Mar 23;13(7):566. doi: 10.3390/cells13070566. Cells. 2024. PMID: 38607005 Free PMC article. Review.
Cited by
-
Pharmacogenetic inhibition of lumbosacral sensory neurons alleviates visceral hypersensitivity in a mouse model of chronic pelvic pain.PLoS One. 2022 Jan 25;17(1):e0262769. doi: 10.1371/journal.pone.0262769. eCollection 2022. PLoS One. 2022. PMID: 35077502 Free PMC article.
-
Molecular and cellular mechanisms of itch and pain in atopic dermatitis and implications for novel therapeutics.Clin Transl Immunology. 2022 May 9;11(5):e1390. doi: 10.1002/cti2.1390. eCollection 2022. Clin Transl Immunology. 2022. PMID: 35582626 Free PMC article. Review.
-
Fibroblastic SMOC2 Suppresses Mechanical Nociception by Inhibiting Coupled Activation of Primary Sensory Neurons.J Neurosci. 2022 May 18;42(20):4069-4086. doi: 10.1523/JNEUROSCI.2132-21.2022. Epub 2022 Apr 18. J Neurosci. 2022. PMID: 35437277 Free PMC article.
-
Activation of NR2A-Wnt-TLR2 Signaling Axis in Satellite Glial Cells of the Dorsal Root Ganglion Contributes to Neuropathic Pain Induced by Nerve Injury in Diabetic Mice.Mol Neurobiol. 2025 Jun;62(6):8013-8037. doi: 10.1007/s12035-025-04754-3. Epub 2025 Feb 18. Mol Neurobiol. 2025. PMID: 39964585
-
Role of Calcium Homeostasis in Modulating EMT in Cancer.Biomedicines. 2021 Sep 11;9(9):1200. doi: 10.3390/biomedicines9091200. Biomedicines. 2021. PMID: 34572386 Free PMC article. Review.
References
-
- Honore P, et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

