DMT analogues: N-ethyl- N-propyl-tryptamine and N-allyl- N-methytryptamine as their hydro-fumarate salts
- PMID: 32843999
- PMCID: PMC7405555
- DOI: 10.1107/S2056989020008683
DMT analogues: N-ethyl- N-propyl-tryptamine and N-allyl- N-methytryptamine as their hydro-fumarate salts
Abstract
The solid-state structures of the hydro-fumarate salts of two N,N-di-alkyl-tryptamines, namely N-ethyl-N-propyl-tryptammonium (EPT) hydro-fumarate {systematic name: [2-(1H-indol-3-yl)eth-yl](meth-yl)propyl-aza-nium 3-carb-oxy-prop-2-enoate}, C15H23N2 +·C4H3O4 -, and N-allyl-N-methyl-tryptammonium (MALT) hydro-fumarate {systematic name: [2-(1H-indol-3-yl)eth-yl](meth-yl)(prop-2-en-1-yl)aza-nium 3-carb-oxy-prop-2-enoate}, C14H19N2 +·C4H3O4 -, are reported. Both compounds possess a protonated tryptammonium cation, and a hydro-fumarate anion in the asymmetric unit. The ethyl group of the EPT cation is modeled as a two-component disorder with 50% occupancy for each component. In the extended structure, N-H⋯O and O-H⋯O hydrogen bonds generate infinite two-dimensional networks parallel to the (001) plane for both compounds.
Keywords: crystal structure; hydrogen bonds; indoles; tryptamines.
© Chadeayne et al. 2020.
Figures

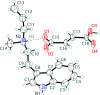
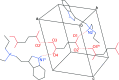
 + y, 1 − z.
+ y, 1 − z.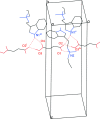
 + y,
+ y,  − z; (vi) −x,
− z; (vi) −x,  + y,
+ y,  − z.
− z.References
-
- Ascic, E., Hansen, C. L., Le Quement, S. T. & Nielsen, T. E. (2012). Chem. Commun. 48, 3345–3347. - PubMed
-
- Brandt, S. D., Freeman, S., Fleet, I. A. & Alder, J. F. (2005b). Analyst, 130, 1258–1262. - PubMed
-
- Brandt, S. D., Freeman, S., Fleet, I. A., McGagh, P. & Alder, J. F. (2005a). Analyst, 130, 330–344. - PubMed
-
- Bruker (2018). APEX3, SAINT, and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
