Di-aqua-bis-{μ-1,5-bis-[(pyridin-2-yl)methyl-idene]carbonohydrazide(1-)}di-μ-chlorido-tetra-chlorido-tetra-zinc(II)
- PMID: 32844027
- PMCID: PMC7405581
- DOI: 10.1107/S2056989020009834
Di-aqua-bis-{μ-1,5-bis-[(pyridin-2-yl)methyl-idene]carbonohydrazide(1-)}di-μ-chlorido-tetra-chlorido-tetra-zinc(II)
Abstract
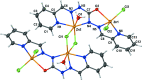
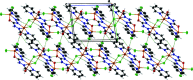
A tetra-nuclear ZnII complex, [Zn4(C13H11N6O)2Cl6(H2O)2] or {[Zn2(HL)(H2O)(Cl2)](μCl)2[Zn2(HL)(H2O)(Cl)]}2, was synthesized by mixing an equimolar amount of a methanol solution containing ZnCl2 and a methanol solution containing the ligand H2 L [1,5-bis-(pyridin-2-yl-methyl-ene)carbono-hydrazide]. In the tetra-nuclear complex, each of the two ligand mol-ecules forms a dinuclear unit that is connected to another dinuclear unit by two bridging chloride anions. In each dinuclear unit, one ZnII cation is penta-coordinated in a N2OCl2 in a distorted square-pyramidal geometry, while the other ZnII cation is hexa-coordinated in a N3OCl2 environment with a distorted octa-hedral geometry. The basal plane around the penta-coordinated ZnII cation is formed by one chloride anion, one oxygen atom, one imino nitro-gen atom and one pyridine nitro-gen atom with the apical position occupied by a chloride anion. The basal plane of the hexa-coordinated ZnII cation is formed by one chloride anion, one hydrazinyl nitro-gen atom, one imino nitro-gen atom and one pyridine nitro-gen atom with the apical positions occupied by a water oxygen atom and a bridged chloro anion from another dinuclear unit, leading to a tetra-nuclear complex. A series of intra-molecular C-H⋯Cl hydrogen bonds is observed in each tetra-nuclear unit. In the crystal, the tetra-nuclear units are connected by inter-molecular C-H⋯Cl, C-H⋯O and N-H⋯O hydrogen bonds, forming a planar two-dimensional structure in the ac plane.
Keywords: complex; crystal structure; square-pyramidal coordination; tetranuclear; zinc.
© Seck et al. 2020.
Figures
References
-
- Addison, A. W., Rao, T. N., Reedijk, J., van Rijn, J. & Verschoor, G. C. (1984). J. Chem. Soc. Dalton Trans. pp. 1349–1356.
-
- Bacchi, A., Carcelli, M., Pelagatti, P., Pelizzi, C., Pelizzi, G. & Zani, F. (1999). J. Inorg. Biochem. 75, 123–133. - PubMed
-
- Bikas, R., Hosseini-Monfared, H., Aleshkevych, P., Szymczak, R., Siczek, M. & Lis, T. (2015). Polyhedron, 88, 48–56.
-
- Dragancea, D., Shova, S., Enyedy, A., Breza, M., Rapta, P., Carrella, L. M., Rentschler, E., Dobrov, A. & Arion, V. B. (2014). Polyhedron, 80, 180–192.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous