Quaternary tryptammonium salts: N, N-dimethyl- N- n-propyl-tryptammonium (DMPT) iodide and N-allyl- N, N-di-methyl-tryptammonium (DMALT) iodide
- PMID: 32844029
- PMCID: PMC7405565
- DOI: 10.1107/S2056989020010014
Quaternary tryptammonium salts: N, N-dimethyl- N- n-propyl-tryptammonium (DMPT) iodide and N-allyl- N, N-di-methyl-tryptammonium (DMALT) iodide
Abstract
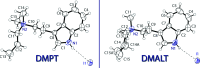
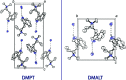
The solid-state structures of two quaternary trytpammonium salts, namely, N,N-dimethyl-N-n-propyl-tryptammonium (DMPT) iodide [systematic name: 2-(1H-indol-3-yl)-N,N-dimethyl-N-propyl-aza-nium iodide], C15H23N2 +·I-, and N-allyl-N,N-di-methyl-tryptammonium (DMALT) iodide, [systematic name: 2-(1H-indol-3-yl)-N,N-dimethyl-N-(prop-2-en-1-yl)aza-nium iodide], C15H21N2 +·I-, are reported. Both salts possess a tri-alkyl-tryptammonium cation and an iodide anion in the asymmetric unit, which are joined together through N-H⋯I inter-actions. The DMALT structure was refined as an inversion twin, and the allyl group is disordered over two orientations with a 0.70 (4):0.30 (4) ratio.
Keywords: crystal structure; hydrogen bonding; indoles; tryptamines.
© Chadeayne et al. 2020.
Figures
References
-
- Arderne, C. & Ndinteh, D. T. (2016). CSD Communication (refcode IZUTUU). CCDC, Cambridge, England.
-
- Bruker (2018). APEX3, SAINT, and SADABS. Bruker AXS Inc., Madison Wisconsin, USA.
-
- Chadeayne, A. R., Golen, J. A. & Manke, D. R. (2019). IUCrData, 4, x190962.
-
- Chadeayne, A. R., Pham, D. N. K., Reid, B. G., Golen, J. A. & Manke, D. R. (2020). ACS Omega, https://doi. org/10.1021/acsomega.0c02208
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
