Dopamine is associated with prioritization of reward-associated memories in Parkinson's disease
- PMID: 32844197
- PMCID: PMC7447515
- DOI: 10.1093/brain/awaa182
Dopamine is associated with prioritization of reward-associated memories in Parkinson's disease
Abstract
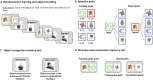
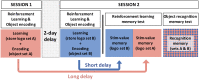
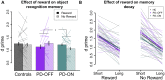
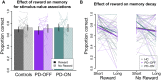
Patients with Parkinson's disease have reduced reward sensitivity related to dopaminergic neuron loss, which is associated with impairments in reinforcement learning. Increasingly, however, dopamine-dependent reward signals are recognized to play an important role beyond reinforcement learning. In particular, it has been shown that reward signals mediated by dopamine help guide the prioritization of events for long-term memory consolidation. Meanwhile, studies of memory in patients with Parkinson's disease have focused on overall memory capacity rather than what is versus what isn't remembered, leaving open questions about the effect of dopamine replacement on the prioritization of memories by reward and the time-dependence of this effect. The current study sought to fill this gap by testing the effect of reward and dopamine on memory in patients with Parkinson's disease. We tested the effect of dopamine modulation and reward on two forms of long-term memory: episodic memory for neutral objects and memory for stimulus-value associations. We measured both forms of memory in a single task, adapting a standard task of reinforcement learning with incidental episodic encoding events of trial-unique objects. Objects were presented on each trial at the time of feedback, which was either rewarding or not. Memory for the trial-unique images and for the stimulus-value associations, and the influence of reward on both, was tested immediately after learning and 2 days later. We measured performance in Parkinson's disease patients tested either ON or OFF their dopaminergic medications and in healthy older control subjects. We found that dopamine was associated with a selective enhancement of memory for reward-associated images, but that it did not influence overall memory capacity. Contrary to predictions, this effect did not differ between the immediate and delayed memory tests. We also found that while dopamine had an effect on reward-modulated episodic memory, there was no effect of dopamine on memory for stimulus-value associations. Our results suggest that impaired prioritization of cognitive resource allocation may contribute to the early cognitive deficits of Parkinson's disease.
Keywords: Parkinson’s disease; dopamine; episodic memory; reinforcement learning; reward.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Differentiating Reinforcement Learning and Episodic Memory in Value-Based Decisions in Parkinson's Disease.J Neurosci. 2025 May 21;45(21):e0911242025. doi: 10.1523/JNEUROSCI.0911-24.2025. J Neurosci. 2025. PMID: 40262901
-
Effects of dopamine on reinforcement learning in Parkinson's disease depend on motor phenotype.Brain. 2020 Dec 5;143(11):3422-3434. doi: 10.1093/brain/awaa335. Brain. 2020. PMID: 33147621 Free PMC article.
-
Dopamine modulates striatal response to reward and punishment in patients with Parkinson's disease: a pharmacological challenge fMRI study.Neuroreport. 2018 May 2;29(7):532-540. doi: 10.1097/WNR.0000000000000970. Neuroreport. 2018. PMID: 29432300 Free PMC article.
-
Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease.Neurosci Biobehav Rev. 2006;30(1):1-23. doi: 10.1016/j.neubiorev.2005.03.024. Epub 2005 Jun 1. Neurosci Biobehav Rev. 2006. PMID: 15935475 Review.
-
Placebo effect and dopamine release.J Neural Transm Suppl. 2006;(70):415-8. doi: 10.1007/978-3-211-45295-0_62. J Neural Transm Suppl. 2006. PMID: 17017561 Review.
Cited by
-
Positive affect modulates memory by regulating the influence of reward prediction errors.Commun Psychol. 2024 Jun 5;2(1):52. doi: 10.1038/s44271-024-00106-4. Commun Psychol. 2024. PMID: 39242805 Free PMC article.
-
Exploring reward-related attention selectivity deficits in Parkinson's disease.Sci Rep. 2021 Sep 21;11(1):18751. doi: 10.1038/s41598-021-97526-7. Sci Rep. 2021. PMID: 34548517 Free PMC article.
-
Dopamine adjusts the circadian gene expression of Per2 and Per3 in human dermal fibroblasts from ADHD patients.J Neural Transm (Vienna). 2021 Jul;128(7):1135-1145. doi: 10.1007/s00702-021-02374-4. Epub 2021 Jul 18. J Neural Transm (Vienna). 2021. PMID: 34275001 Free PMC article.
-
Sleep spindle density and temporal clustering are associated with sleep-dependent memory consolidation in Parkinson's disease.J Clin Sleep Med. 2024 Jul 1;20(7):1153-1162. doi: 10.5664/jcsm.11080. J Clin Sleep Med. 2024. PMID: 38427318 Free PMC article.
-
Effects of Pramipexole Combined with Nerve Growth Factor on Cognitive Impairment and Urinary AD7c-NTP Expression in Patients with Parkinson's Disease.Comput Math Methods Med. 2022 Apr 26;2022:3398732. doi: 10.1155/2022/3398732. eCollection 2022. Comput Math Methods Med. 2022. Retraction in: Comput Math Methods Med. 2023 Jun 28;2023:9787324. doi: 10.1155/2023/9787324. PMID: 35516456 Free PMC article. Retracted.
References
-
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli J.. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron 2006; 50: 507–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

