Pooling Upper Respiratory Specimens for Rapid Mass Screening of COVID-19 by Real-Time RT-PCR
- PMID: 32844739
- PMCID: PMC7510748
- DOI: 10.3201/eid2610.201955
Pooling Upper Respiratory Specimens for Rapid Mass Screening of COVID-19 by Real-Time RT-PCR
Abstract
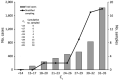
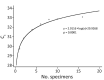
To validate the specimen-pooling strategy for real-time reverse transcription PCR detection of severe acute respiratory syndrome coronavirus 2, we generated different pools including positive specimens, reflecting the distribution of cycle threshold values at initial diagnosis. Cumulative sensitivities of tested pool sizes suggest pooling of <6 specimens for surveillance by this method.
Keywords: COVID-19; SAR-CoV-2; SARS; SARS-CoV-2; SARS-related coronavirus; South Korea; coronavirus disease; real-time PCR; respiratory infections; reverse transcription PCR; severe acute respiratory syndrome; severe acute respiratory syndrome coronavirus 2; specimen pooling; viruses; zoonoses.
Figures
Similar articles
-
Evaluation of performance of two SARS-CoV-2 Rapid IgM-IgG combined antibody tests on capillary whole blood samples from the fingertip.PLoS One. 2020 Sep 17;15(9):e0237694. doi: 10.1371/journal.pone.0237694. eCollection 2020. PLoS One. 2020. PMID: 32941461 Free PMC article.
-
A novel strategy for community screening of SARS-CoV-2 (COVID-19): Sample pooling method.PLoS One. 2020 Aug 28;15(8):e0238417. doi: 10.1371/journal.pone.0238417. eCollection 2020. PLoS One. 2020. PMID: 32857823 Free PMC article.
-
A reverse-transcription recombinase-aided amplification assay for the rapid detection of N gene of severe acute respiratory syndrome coronavirus 2(SARS-CoV-2).Virology. 2020 Oct;549:1-4. doi: 10.1016/j.virol.2020.07.006. Epub 2020 Jul 29. Virology. 2020. PMID: 32758712 Free PMC article.
-
Guidelines for Laboratory Diagnosis of Coronavirus Disease 2019 (COVID-19) in Korea.Ann Lab Med. 2020 Sep;40(5):351-360. doi: 10.3343/alm.2020.40.5.351. Ann Lab Med. 2020. PMID: 32237288 Free PMC article. Review.
-
[SARS-CoV-2 and Microbiological Diagnostic Dynamics in COVID-19 Pandemic].Mikrobiyol Bul. 2020 Jul;54(3):497-509. doi: 10.5578/mb.69839. Mikrobiyol Bul. 2020. PMID: 32755524 Review. Turkish.
Cited by
-
Comparative evaluation of a dual-target real-time RT-PCR assay for COVID-19 diagnosis and assessment of performance in pooled saliva and nasopharyngeal swab samples.Expert Rev Mol Diagn. 2021 Jul;21(7):741-747. doi: 10.1080/14737159.2021.1933445. Epub 2021 Jun 4. Expert Rev Mol Diagn. 2021. PMID: 34014785 Free PMC article.
-
Evaluation of Sample Pooling for SARS-CoV-2 Detection in Nasopharyngeal Swab and Saliva Samples with the Idylla SARS-CoV-2 Test.Microbiol Spectr. 2021 Dec 22;9(3):e0099621. doi: 10.1128/Spectrum.00996-21. Epub 2021 Nov 10. Microbiol Spectr. 2021. PMID: 34756076 Free PMC article.
-
Screening multi-dimensional heterogeneous populations for infectious diseases under scarce testing resources, with application to COVID-19.Nav Res Logist. 2022 Feb;69(1):3-20. doi: 10.1002/nav.21985. Epub 2021 Mar 16. Nav Res Logist. 2022. PMID: 38607835 Free PMC article.
-
Analysis of current SARS-CoV-2 infection in a large population of blood donors evidenced that RNAemia is rare in plasma.Transfusion. 2021 Jul;61(7):2137-2145. doi: 10.1111/trf.16406. Epub 2021 Jun 10. Transfusion. 2021. PMID: 33860542 Free PMC article.
-
Comparison of the Clinical Performance of the Point-of-care STANDARD M10 SARS-CoV-2 and Xpert Xpress SARS-CoV-2 Assays.Ann Lab Med. 2023 Jan 1;43(1):111-113. doi: 10.3343/alm.2023.43.1.111. Epub 2022 Sep 1. Ann Lab Med. 2023. PMID: 36045067 Free PMC article. No abstract available.
References
-
- World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19—11 March 2020. Geneva: The Organization; 2020. [cited 2020 Aug 19]. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

