The JAK-STAT pathway regulates CD38 on myeloma cells in the bone marrow microenvironment: therapeutic implications
- PMID: 32844992
- PMCID: PMC7702477
- DOI: 10.1182/blood.2019004332
The JAK-STAT pathway regulates CD38 on myeloma cells in the bone marrow microenvironment: therapeutic implications
Abstract
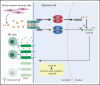
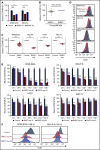
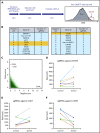
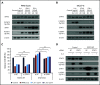
Anti-CD38 monoclonal antibody (MoAb) treatments including daratumumab (DARA) are effective therapies for both newly diagnosed and relapsed multiple myeloma (MM). In this study, we examined the soluble factors that modulate CD38 expression and are associated with sensitivity to DARA-mediated antibody-dependent cellular cytotoxicity (ADCC) in the bone marrow (BM) microenvironment. Importantly, primary BM stromal cell (BMSC) culture supernatant (BMSC-sup) and interleukin-6 (IL-6) downregulated CD38 expression and reduced DARA-mediated ADCC. Both cytokine profiling of the BMSC-sup and genome-scale clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (Cas9) knockout screening in MM cell lines identified and validated the JAK-STAT3 signaling pathway mediating CD38 downregulation, whereas the JAK-STAT1 pathway mediated CD38 upregulation. STAT3 knockdown abrogated BMSC-sup- and IL-6-induced CD38 downregulation on MM cell lines. We also confirmed that STAT3 and CD38 is negatively correlated in primary MM cells. To assess potential clinical relevance, pharmacological inhibition of the JAK-STAT pathway on BMSC-sup-induced CD38 downregulation was further examined. JAK inhibitor ruxolitinib inhibited STAT3 phosphorylation in MM cell lines, upregulated CD38 expression in MM cell lines and primary patient MM cells, and augmented DARA-mediated ADCC against MM cell lines. Taken together, our results suggest that CD38 expression on MM cells in the BM microenvironment is regulated by both STAT1 (positively) and STAT3 (negatively), and that inhibition of the JAK-STAT3 pathway represents a novel therapeutic option to enhance CD38 expression and anti-CD38 MoAb-mediated MM cytotoxicity.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: K.C.A. serves on advisory boards to Celgene, Millennium-Takeda, Janssen, Sanofi-Aventis, Bristol Myers Squibb, Gilead, Precision Biosciences, and Tolero, and is a scientific founder of OncoPep and C4 Therapeutics. The remaining authors declare no competing financial interests.
Figures


Comment in
-
We need CD38 STAT-JAK.Blood. 2020 Nov 12;136(20):2246-2248. doi: 10.1182/blood.2020007467. Blood. 2020. PMID: 33180919 No abstract available.
References
-
- Kumar SK, Anderson KC. Immune therapies in multiple myeloma. Clin Cancer Res. 2016;22(22):5453-5460. - PubMed
-
- Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394(10192):29-38. - PubMed
-
- Mateos MV, Dimopoulos MA, Cavo M, et al. ; ALCYONE Trial Investigators . Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378(6):518-528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

