A New Mechanically-Interlocked [Pd2 L4 ] Cage Motif by Dimerization of two Peptide-based Lemniscates
- PMID: 32845570
- PMCID: PMC7756597
- DOI: 10.1002/anie.202010995
A New Mechanically-Interlocked [Pd2 L4 ] Cage Motif by Dimerization of two Peptide-based Lemniscates
Abstract
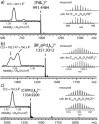
Most metallo-supramolecular assemblies of low nuclearity adopt simple topologies, with bridging ligands spanning neighboring metal centers in a direct fashion. Here we contribute a new structural motif to the family of host compounds with low metal count (two) that consists of a pair of doubly-interlocked, Figure-eight-shaped subunits, also termed "lemniscates". Each metal is chelated by two chiral bidentate ligands, composed of a peptidic macrocycle that resembles a natural product with two pyridyl-terminated arms. DFT calculation results suggest that dimerization of the mononuclear halves is driven by a combination of 1) Coulomb interaction with a central anion, 2) π-stacking between intertwined ligand arms and 3) dispersive interactions between the structure's compact inner core bedded into an outer shell composed of the cavitand-type macrocycles. The resulting cage-like architecture was characterized by NMR, MS and X-ray structure analyses. This new mechanically bonded system highlights the scope of structural variety accessible in metal-mediated self-assemblies composed of only a few constituents.
Keywords: catenane; chirality; coordination cage; mechanical bond; supramolecular chemistry.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

