NuMA interaction with chromatin is vital for proper chromosome decondensation at the mitotic exit
- PMID: 32845810
- PMCID: PMC7851854
- DOI: 10.1091/mbc.E20-06-0415
NuMA interaction with chromatin is vital for proper chromosome decondensation at the mitotic exit
Abstract
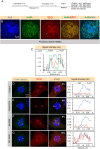
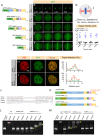
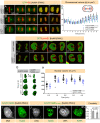
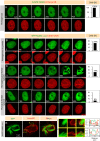
NuMA is an abundant long coiled-coil protein that plays a prominent role in spindle organization during mitosis. In interphase, NuMA is localized to the nucleus and hypothesized to control gene expression and chromatin organization. However, because of the prominent mitotic phenotype upon NuMA loss, its precise function in the interphase nucleus remains elusive. Here, we report that NuMA is associated with chromatin in interphase and prophase but released upon nuclear envelope breakdown (NEBD) by the action of Cdk1. We uncover that NuMA directly interacts with DNA via evolutionarily conserved sequences in its C-terminus. Notably, the expression of the DNA-binding-deficient mutant of NuMA affects chromatin decondensation at the mitotic exit, and nuclear shape in interphase. We show that the nuclear shape defects observed upon mutant NuMA expression are due to its potential to polymerize into higher-order fibrillar structures. Overall, this work establishes the spindle-independent function of NuMA in choreographing proper chromatin decompaction and nuclear shape by directly associating with the DNA.
Figures

Similar articles
-
The mitotic protein NuMA plays a spindle-independent role in nuclear formation and mechanics.J Cell Biol. 2020 Dec 7;219(12):e202004202. doi: 10.1083/jcb.202004202. J Cell Biol. 2020. PMID: 33044554 Free PMC article.
-
Nuclear Mitotic Apparatus (NuMA) Interacts with and Regulates Astrin at the Mitotic Spindle.J Biol Chem. 2016 Sep 16;291(38):20055-67. doi: 10.1074/jbc.M116.724831. Epub 2016 Jul 26. J Biol Chem. 2016. PMID: 27462074 Free PMC article.
-
NuMA phosphorylation by CDK1 couples mitotic progression with cortical dynein function.EMBO J. 2013 Sep 11;32(18):2517-29. doi: 10.1038/emboj.2013.172. Epub 2013 Aug 6. EMBO J. 2013. PMID: 23921553 Free PMC article.
-
Role of NuMA in vertebrate cells: review of an intriguing multifunctional protein.Front Biosci. 2006 Jan 1;11:1137-46. doi: 10.2741/1868. Front Biosci. 2006. PMID: 16146802 Review.
-
NuMA after 30 years: the matrix revisited.Trends Cell Biol. 2010 Apr;20(4):214-22. doi: 10.1016/j.tcb.2010.01.003. Trends Cell Biol. 2010. PMID: 20137953 Free PMC article. Review.
Cited by
-
Dephosphorylation in nuclear reassembly after mitosis.Front Cell Dev Biol. 2022 Oct 4;10:1012768. doi: 10.3389/fcell.2022.1012768. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36268509 Free PMC article. Review.
-
NuMA mechanically reinforces the spindle independently of its partner dynein.bioRxiv [Preprint]. 2024 Dec 1:2024.11.29.622360. doi: 10.1101/2024.11.29.622360. bioRxiv. 2024. Update in: Curr Biol. 2025 Jul 30:S0960-9822(25)00894-2. doi: 10.1016/j.cub.2025.07.028. PMID: 40741481 Free PMC article. Updated. Preprint.
-
NuMA mechanically reinforces the spindle independently of its partner dynein.Curr Biol. 2025 Jul 30:S0960-9822(25)00894-2. doi: 10.1016/j.cub.2025.07.028. Online ahead of print. Curr Biol. 2025. PMID: 40769151
-
The Nuclear Mitotic Apparatus (NuMA) Protein: A Key Player for Nuclear Formation, Spindle Assembly, and Spindle Positioning.Front Cell Dev Biol. 2021 Apr 1;9:653801. doi: 10.3389/fcell.2021.653801. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33869212 Free PMC article. Review.
-
Membrane compartmentalization of Ect2/Cyk4/Mklp1 and NuMA/dynein regulates cleavage furrow formation.J Cell Biol. 2022 Dec 5;221(12):e202203127. doi: 10.1083/jcb.202203127. Epub 2022 Oct 5. J Cell Biol. 2022. PMID: 36197340 Free PMC article.
References
-
- Antonin W, Neumann H (2016). Chromosome condensation and decondensation during mitosis. Curr Opin Cell Biol , 15–22. - PubMed
-
- Baarlink C, Plessner M, Sherrard A, Morita K, Misu S, Virant D, Kleinschnitz EM, Harniman R, Alibhai D, Baumeister S, et al. (2017). A transient pool of nuclear F-actin at mitotic exit controls chromatin organization. Nat Cell Biol , 1389–1399. - PubMed
-
- Batty P, Gerlich DW (2019). Mitotic chromosome mechanics: how cells segregate their genome. Trends Cell Biol , 717–726. - PubMed
-
- Chandramouly G, Abad PC, Knowles DW, Lelièvre SA (2007). The control of tissue architecture over nuclear organization is crucial for epithelial cell fate. J Cell Sci , 1596–1606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

