Cherenkov emissions for studying tumor changes during radiation therapy: An exploratory study in domesticated dogs with naturally-occurring cancer
- PMID: 32845905
- PMCID: PMC7449466
- DOI: 10.1371/journal.pone.0238106
Cherenkov emissions for studying tumor changes during radiation therapy: An exploratory study in domesticated dogs with naturally-occurring cancer
Abstract
Purpose: Real-time monitoring of physiological changes of tumor tissue during radiation therapy (RT) could improve therapeutic efficacy and predict therapeutic outcomes. Cherenkov radiation is a normal byproduct of radiation deposited in tissue. Previous studies in rat tumors have confirmed a correlation between Cherenkov emission spectra and optical measurements of blood-oxygen saturation based on the tissue absorption coefficients. The purpose of this study is to determine if it is feasible to image Cherenkov emissions during radiation therapy in larger human-sized tumors of pet dogs with cancer. We also wished to validate the prior work in rats, to determine if Cherenkov emissions have the potential to act an indicator of blood-oxygen saturation or water-content changes in the tumor tissue-both of which have been correlated with patient prognosis.
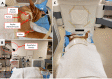
Methods: A DoseOptics camera, built to image the low-intensity emission of Cherenkov radiation, was used to measure Cherenkov intensities in a cohort of cancer-bearing pet dogs during clinical irradiation. Tumor type and location varied, as did the radiation fractionation scheme and beam arrangement, each planned according to institutional standard-of-care. Unmodulated radiation was delivered using multiple 6 MV X-ray beams from a clinical linear accelerator. Each dog was treated with a minimum of 16 Gy total, in ≥3 fractions. Each fraction was split into at least three subfractions per gantry angle. During each subfraction, Cherenkov emissions were imaged.
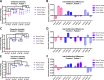
Results: We documented significant intra-subfraction differences between the Cherenkov intensities for normal tissue, whole-tumor tissue, tissue at the edge of the tumor and tissue at the center of the tumor (p<0.05). Additionally, intra-subfraction changes suggest that Cherenkov emissions may have captured fluctuating absorption properties within the tumor.
Conclusion: Here we demonstrate that it is possible to obtain Cherenkov emissions from canine cancers within a fraction of radiotherapy. The entire optical spectrum was obtained which includes the window for imaging changes in water and hemoglobin saturation. This lends credence to the goal of using this method during radiotherapy in human patients and client-owned pets.
Conflict of interest statement
XZ is currently employed by Artificial Intelligence, Marchex Inc. However, XZ was employed solely by Duke University at the time of his involvement with this research. There are no patents, products in development, or marketed products to declare. This does not alter our adherence to PLOS ONE's policies on data or materials sharing.
Figures




Similar articles
-
Initial Clinical Experience of Cherenkov Imaging in External Beam Radiation Therapy Identifies Opportunities to Improve Treatment Delivery.Int J Radiat Oncol Biol Phys. 2021 Apr 1;109(5):1627-1637. doi: 10.1016/j.ijrobp.2020.11.013. Epub 2020 Nov 20. Int J Radiat Oncol Biol Phys. 2021. PMID: 33227443 Free PMC article.
-
An investigation of clinical treatment field delivery verification using cherenkov imaging: IMRT positioning shifts and field matching.Med Phys. 2019 Jan;46(1):302-317. doi: 10.1002/mp.13250. Epub 2018 Nov 19. Med Phys. 2019. PMID: 30346639
-
Imaging radiation dose in breast radiotherapy by X-ray CT calibration of Cherenkov light.Nat Commun. 2020 May 8;11(1):2298. doi: 10.1038/s41467-020-16031-z. Nat Commun. 2020. PMID: 32385233 Free PMC article.
-
Positron emission tomography for radiation treatment planning.Strahlenther Onkol. 2005 Aug;181(8):483-99. doi: 10.1007/s00066-005-1422-7. Strahlenther Onkol. 2005. PMID: 16044216 Review.
-
Application of Cherenkov radiation in tumor imaging and treatment.Future Oncol. 2022 Sep;18(27):3101-3118. doi: 10.2217/fon-2022-0022. Epub 2022 Sep 6. Future Oncol. 2022. PMID: 36065976 Review.
Cited by
-
Cherenkov light emission in external beam radiation therapy of the larynx.J Biomed Opt. 2025 May;30(5):055002. doi: 10.1117/1.JBO.30.5.055002. Epub 2025 May 29. J Biomed Opt. 2025. PMID: 40444263 Free PMC article.
-
FLASH Radiotherapy and the Use of Radiation Dosimeters.Cancers (Basel). 2023 Jul 30;15(15):3883. doi: 10.3390/cancers15153883. Cancers (Basel). 2023. PMID: 37568699 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

