Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer
- PMID: 32846060
- PMCID: PMC7506467
- DOI: 10.1056/NEJMoa2005653
Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer
Abstract
Background: RET fusions are oncogenic drivers in 1 to 2% of non-small-cell lung cancers (NSCLCs). In patients with RET fusion-positive NSCLC, the efficacy and safety of selective RET inhibition are unknown.
Methods: We enrolled patients with advanced RET fusion-positive NSCLC who had previously received platinum-based chemotherapy and those who were previously untreated separately in a phase 1-2 trial of selpercatinib. The primary end point was an objective response (a complete or partial response) as determined by an independent review committee. Secondary end points included the duration of response, progression-free survival, and safety.
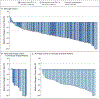
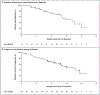
Results: In the first 105 consecutively enrolled patients with RET fusion-positive NSCLC who had previously received at least platinum-based chemotherapy, the percentage with an objective response was 64% (95% confidence interval [CI], 54 to 73). The median duration of response was 17.5 months (95% CI, 12.0 to could not be evaluated), and 63% of the responses were ongoing at a median follow-up of 12.1 months. Among 39 previously untreated patients, the percentage with an objective response was 85% (95% CI, 70 to 94), and 90% of the responses were ongoing at 6 months. Among 11 patients with measurable central nervous system metastasis at enrollment, the percentage with an objective intracranial response was 91% (95% CI, 59 to 100). The most common adverse events of grade 3 or higher were hypertension (in 14% of the patients), an increased alanine aminotransferase level (in 12%), an increased aspartate aminotransferase level (in 10%), hyponatremia (in 6%), and lymphopenia (in 6%). A total of 12 of 531 patients (2%) discontinued selpercatinib because of a drug-related adverse event.
Conclusions: Selpercatinib had durable efficacy, including intracranial activity, with mainly low-grade toxic effects in patients with RET fusion-positive NSCLC who had previously received platinum-based chemotherapy and those who were previously untreated. (Funded by Loxo Oncology and others; LIBRETTO-001 ClinicalTrials.gov number, NCT03157128.).
Copyright © 2020 Massachusetts Medical Society.
Figures
Comment in
-
Selpercatinib Aimed at RET-Altered Cancers.N Engl J Med. 2020 Aug 27;383(9):868-869. doi: 10.1056/NEJMe2024831. N Engl J Med. 2020. PMID: 32846067 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
