'Liking' and 'wanting' in eating and food reward: Brain mechanisms and clinical implications
- PMID: 32846152
- PMCID: PMC7655589
- DOI: 10.1016/j.physbeh.2020.113152
'Liking' and 'wanting' in eating and food reward: Brain mechanisms and clinical implications
Abstract
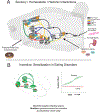
It is becoming clearer how neurobiological mechanisms generate 'liking' and 'wanting' components of food reward. Mesocorticolimbic mechanisms that enhance 'liking' include brain hedonic hotspots, which are specialized subregions that are uniquely able to causally amplify the hedonic impact of palatable tastes. Hedonic hotspots are found in nucleus accumbens medial shell, ventral pallidum, orbitofrontal cortex, insula cortex, and brainstem. In turn, a much larger mesocorticolimbic circuitry generates 'wanting' or incentive motivation to obtain and consume food rewards. Hedonic and motivational circuitry interact together and with hypothalamic homeostatic circuitry, allowing relevant physiological hunger and satiety states to modulate 'liking' and 'wanting' for food rewards. In some conditions such as drug addiction, 'wanting' is known to dramatically detach from 'liking' for the same reward, and this may also occur in over-eating disorders. Via incentive sensitization, 'wanting' selectively becomes higher, especially when triggered by reward cues when encountered in vulnerable states of stress, etc. Emerging evidence suggests that some cases of obesity and binge eating disorders may reflect an incentive-sensitization brain signature of cue hyper-reactivity, causing excessive 'wanting' to eat. Future findings on the neurobiological bases of 'liking' and 'wanting' can continue to improve understanding of both normal food reward and causes of clinical eating disorders.
Keywords: Feeding; Nucleus accumbens; Prefrontal cortex; Ventral pallidum; ‘Liking’; ‘Wanting’.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare no competing financial interests.
Figures



References
-
- Devoto F, Zapparoli L, Bonandrini R, Berlingeri M, Ferrulli A, Luzi L, Banfi G, Paulesu E, Hungry brains: A meta-analytical review of brain activation imaging studies on food perception and appetite in obese individuals, Neurosci. Biobehav. Rev 94 (2018) 271–285, 10.1016/j.neubiorev.2018.07.017. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

