Phase Separation of a PKA Regulatory Subunit Controls cAMP Compartmentation and Oncogenic Signaling
- PMID: 32846158
- PMCID: PMC7502557
- DOI: 10.1016/j.cell.2020.07.043
Phase Separation of a PKA Regulatory Subunit Controls cAMP Compartmentation and Oncogenic Signaling
Abstract
The fidelity of intracellular signaling hinges on the organization of dynamic activity architectures. Spatial compartmentation was first proposed over 30 years ago to explain how diverse G protein-coupled receptors achieve specificity despite converging on a ubiquitous messenger, cyclic adenosine monophosphate (cAMP). However, the mechanisms responsible for spatially constraining this diffusible messenger remain elusive. Here, we reveal that the type I regulatory subunit of cAMP-dependent protein kinase (PKA), RIα, undergoes liquid-liquid phase separation (LLPS) as a function of cAMP signaling to form biomolecular condensates enriched in cAMP and PKA activity, critical for effective cAMP compartmentation. We further show that a PKA fusion oncoprotein associated with an atypical liver cancer potently blocks RIα LLPS and induces aberrant cAMP signaling. Loss of RIα LLPS in normal cells increases cell proliferation and induces cell transformation. Our work reveals LLPS as a principal organizer of signaling compartments and highlights the pathological consequences of dysregulating this activity architecture.
Keywords: DnaJB1-PKA; FLC; FRET; biosensor; fibrolamellar carcinoma; live cell imaging; membraneless organelle; signal transduction; split GFP.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures
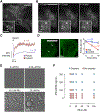
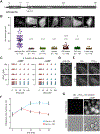
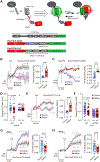


Comment in
-
cAMP Signaling in Nanodomains.Cell. 2020 Sep 17;182(6):1379-1381. doi: 10.1016/j.cell.2020.08.041. Cell. 2020. PMID: 32946779
-
Phase separation of protein kinase A: a new paradigm in cardiac regulation?Nat Rev Cardiol. 2024 Aug;21(8):523. doi: 10.1038/s41569-024-01048-4. Nat Rev Cardiol. 2024. PMID: 38839881 No abstract available.
References
-
- Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, and Tsien RY (1993). Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science 260, 222–226. - PubMed
-
- Baillie GS (2009). Compartmentalized signalling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J 276, 1790–1799. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

