COVID-19: Pathophysiology, treatment options, nanotechnology approaches, and research agenda to combating the SARS-CoV2 pandemic
- PMID: 32846164
- PMCID: PMC7443335
- DOI: 10.1016/j.lfs.2020.118336
COVID-19: Pathophysiology, treatment options, nanotechnology approaches, and research agenda to combating the SARS-CoV2 pandemic
Abstract
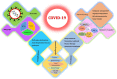
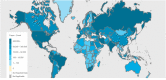
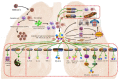
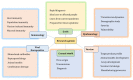
The recent corona virus disease (COVID-19) outbreak has claimed the lives of many around the world and highlighted an urgent need for experimental strategies to prevent, treat and eradicate the virus. COVID-19, an infectious disease caused by a novel corona virus and no approved specific treatment is available yet. A vast number of promising antiviral treatments involving nanotechnology are currently under investigation to aid in the development of COVID-19 drug delivery. The prospective treatment options integrating the ever-expanding field of nanotechnology have been compiled, with the objective to show that these can be potentially developed for COVID-19 treatment. This review summarized the current state of knowledge, research priorities regarding the pandemic and post COVID-19. We also focus on the possible nanotechnology approaches that have proven to be successful against other viruses and the research agenda to combat COVID-19.
Keywords: COVID-19; Nanotechnology; Post COVID-19; Research agenda; Therapeutics treatment.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
No conflict of interest was reported by the authors of this article.
Figures


References
-
- Clinical management of COVID-19, (n.d). https://www.who.int/publications/i/item/clinical-management-of-covid-19 (accessed June 11, 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

