Prospect of SARS-CoV-2 spike protein: Potential role in vaccine and therapeutic development
- PMID: 32846196
- PMCID: PMC7443330
- DOI: 10.1016/j.virusres.2020.198141
Prospect of SARS-CoV-2 spike protein: Potential role in vaccine and therapeutic development
Abstract
The recent outbreak of the betacoronavirus SARS-CoV-2 has become a significant concern to public health care worldwide. As of August 19, 2020, more than 22,140,472 people are infected, and over 781,135 people have died due to this deadly virus. In the USA alone, over 5,482,602 people are currently infected, and more than 171,823 people have died. SARS-CoV-2 has shown a higher infectivity rate and a more extended incubation period as compared to previous coronaviruses. SARS-CoV-2 binds much more strongly than SARS-CoV to the same host receptor, angiotensin-converting enzyme 2 (ACE2). Previously, several methods to develop a vaccine against SARS-CoV or MERS-CoV have been tried with limited success. Since SARS-CoV-2 uses the spike (S) protein for entry to the host cell, it is one of the most preferred targets for making vaccines or therapeutics against SARS-CoV-2. In this review, we have summarised the characteristics of the S protein, as well as the different approaches being used for the development of vaccines and/or therapeutics based on the S protein.
Keywords: COVID-19; SARS-CoV-2; Spike protein; Therapeutic; Vaccine.
Copyright © 2020. Published by Elsevier B.V.
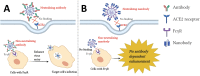
Figures

References
-
- ADPVAC BARBARIAN NORDIC Enters Agreement With ADAPVAC to Advance COVID-19 Vaccine Program. May 6. 2020. https://www.adaptvac.com/news Accesses May 21, 2020., n.d.
-
- AJ Vaccines AJ Vaccines to Develop Vaccine for COVID-19. March 06. 2020. https://ajvaccines.com/news/ Accessed May 21, 2020., n.d.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

