The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity
- PMID: 32846426
- PMCID: PMC8435934
- DOI: 10.1038/s41586-020-2702-1
The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity
Erratum in
-
Publisher Correction: The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity.Nature. 2020 Dec;588(7836):E4. doi: 10.1038/s41586-020-2954-9. Nature. 2020. PMID: 33199921
Abstract
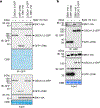
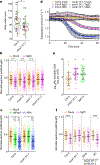
Perception of biotic and abiotic stresses often leads to stomatal closure in plants1,2. Rapid influx of calcium ions (Ca2+) across the plasma membrane has an important role in this response, but the identity of the Ca2+ channels involved has remained elusive3,4. Here we report that the Arabidopsis thaliana Ca2+-permeable channel OSCA1.3 controls stomatal closure during immune signalling. OSCA1.3 is rapidly phosphorylated upon perception of pathogen-associated molecular patterns (PAMPs). Biochemical and quantitative phosphoproteomics analyses reveal that the immune receptor-associated cytosolic kinase BIK1 interacts with and phosphorylates the N-terminal cytosolic loop of OSCA1.3 within minutes of treatment with the peptidic PAMP flg22, which is derived from bacterial flagellin. Genetic and electrophysiological data reveal that OSCA1.3 is permeable to Ca2+, and that BIK1-mediated phosphorylation on its N terminus increases this channel activity. Notably, OSCA1.3 and its phosphorylation by BIK1 are critical for stomatal closure during immune signalling, and OSCA1.3 does not regulate stomatal closure upon perception of abscisic acid-a plant hormone associated with abiotic stresses. This study thus identifies a plant Ca2+ channel and its activation mechanisms underlying stomatal closure during immune signalling, and suggests specificity in Ca2+ influx mechanisms in response to different stresses.
Conflict of interest statement
Figures








Comment in
-
Calcium channel in plants helps shut the door on intruders.Nature. 2020 Sep;585(7826):507-508. doi: 10.1038/d41586-020-02504-0. Nature. 2020. PMID: 32895562 No abstract available.
References
-
- Sussmilch FC, Schultz J, Hedrich R & Roelfsema MRG Acquiring control: the evolution of stomatal signalling pathways. Trends Plant Sci. 24, 342–351 (2019). - PubMed
-
- Hedrich R Ion channels in plants. Physiol. Rev 92, 1777–1811 (2012). - PubMed
-
- Berridge MJ, Lipp P & Bootman MD The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol 1, 11–21 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

