Sephin1 Protects Neurons against Excitotoxicity Independently of the Integrated Stress Response
- PMID: 32846985
- PMCID: PMC7504470
- DOI: 10.3390/ijms21176088
Sephin1 Protects Neurons against Excitotoxicity Independently of the Integrated Stress Response
Abstract
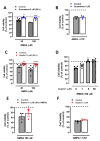
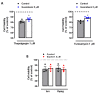
Sephin1 is a derivative of guanabenz that inhibits the dephosphorylation of the eukaryotic initiation factor 2 alpha (eIF2α) and therefore may enhance the integrated stress response (ISR), an adaptive mechanism against different cellular stresses, such as accumulation of misfolded proteins. Unlike guanabenz, Sephin1 provides neuroprotection without adverse effects on the α2-adrenergic system and therefore it is considered a promising pharmacological therapeutic tool. Here, we have studied the effects of Sephin1 on N-methyl-D-aspartic acid (NMDA) receptor signaling which may modulate the ISR and contribute to excitotoxic neuronal loss in several neurodegenerative conditions. Time-course analysis of peIF2α levels after NMDA receptor overactivation showed a delayed dephosphorylation that occurred in the absence of activating transcription factor 4 (ATF4) and therefore independently of the ISR, in contrast to that observed during endoplasmic reticulum (ER) stress induced by tunicamycin and thapsigargin. Similar to guanabenz, Sephin1 completely blocked NMDA-induced neuronal death and was ineffective against AMPA-induced excitotoxicity, whereas it did not protect from experimental ER stress. Interestingly, both guanabenz and Sephin1 partially but significantly reduced NMDA-induced cytosolic Ca2+ increase, leading to a complete inhibition of subsequent calpain activation. We conclude that Sephin1 and guanabenz share common strong anti-excitotoxic properties with therapeutic potential unrelated to the ISR.
Keywords: NMDA; Sephin1; calcium; calpain; excitotoxicity; guanabenz; integrated stress response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

