Alkoxyamines Designed as Potential Drugs against Plasmodium and Schistosoma Parasites
- PMID: 32846996
- PMCID: PMC7503767
- DOI: 10.3390/molecules25173838
Alkoxyamines Designed as Potential Drugs against Plasmodium and Schistosoma Parasites
Abstract
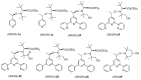
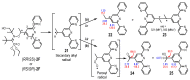
Malaria and schistosomiasis are major infectious causes of morbidity and mortality in the tropical and sub-tropical areas. Due to the widespread drug resistance of the parasites, the availability of new efficient and affordable drugs for these endemic pathologies is now a critical public health issue. In this study, we report the design, the synthesis and the preliminary biological evaluation of a series of alkoxyamine derivatives as potential drugs against Plasmodium and Schistosoma parasites. The compounds (RS/SR)-2F, (RR/SS)-2F, and 8F, having IC50 values in nanomolar range against drug-resistant P. falciparum strains, but also five other alkoxyamines, inducing the death of all adult worms of S. mansoni in only 1 h, can be considered as interesting chemical starting points of the series for improvement of the activity, and further structure activity, relationship studies. Moreover, investigation of the mode of action and the rate constants kd for C-ON bond homolysis of new alkoxyamines is reported, showing a possible alkyl radical mediated biological activity. A theoretical chemistry study allowed us to design new structures of alkoxyamines in order to improve the selectivity index of these drugs.
Keywords: alkoxyamine; alkylation; heme; malaria; radical chemistry; schistosomiasis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
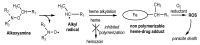




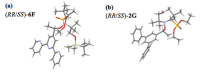


References
-
- World Health Organization . World Malaria Report 2019. World Health Organization; Geneva, Switzerland: 2019. p. 185.
-
- World Health Organization Artemisinin and Artemisinin-Based Combination Therapy Resistance: Status Report; 2016. [(accessed on 13 August 2020)]; Available online: https://apps.who.int/iris/bitstream/handle/10665/250294/WHO-HTM-GMP-2016....
-
- Duru V., Khim N., Leang R., Kim S., Domergue A., Kloeung N., Ke S., Chy S., Eam R., Khean C., et al. Plasmodium falciparum dihydroartemisinin-piperaquine failures in Cambodia are associated with mutant K13 parasites presenting high survival rates in novel piperaquine in vitro assays: Retrospective and prospective investigations. BMC Med. 2015;13:305. doi: 10.1186/s12916-015-0539-5. - DOI - PMC - PubMed
-
- Witkowski B., Duru V., Khim N., Ross L.S., Saintpierre B., Beghain J., Chy S., Kim S., Ke S., Kloeung N., et al. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: A phenotype-genotype association study. Lancet Infect. Dis. 2017;17:174–183. doi: 10.1016/S1473-3099(16)30415-7. - DOI - PMC - PubMed
-
- Ménard S., Ben Haddou T., Ramadani A.P., Ariey F., Iriart X., Beghain J., Bouchier C., Witkowski B., Berry A., Mercereau-Puijalon O., et al. Induction of multidrug tolerance in Plasmodium falciparum by extended artemisinin pressure. Emerg. Infect. Dis. 2015;21:1733–1741. doi: 10.3201/eid2110.150682. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

