Health Technology Assessment Report on Vagus Nerve Stimulation in Drug-Resistant Epilepsy
- PMID: 32847092
- PMCID: PMC7504285
- DOI: 10.3390/ijerph17176150
Health Technology Assessment Report on Vagus Nerve Stimulation in Drug-Resistant Epilepsy
Abstract
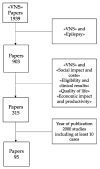
Background: Vagus nerve stimulation (VNS) is a palliative treatment for medical intractable epileptic syndromes not eligible for resective surgery. Health technology assessment (HTA) represents a modern approach to the analysis of technologies used for healthcare. The purpose of this study is to assess the clinical, organizational, financial, and economic impact of VNS therapy in drug-resistant epilepsies and to establish the congruity between costs incurred and health service reimbursement. Methods: The present study used an HTA approach. It is based on an extensive detailed bibliographic search on databases (Medline, Pubmed, Embase and Cochrane, sites of scientific societies and institutional sites). The HTA study includes the following issues: (a) social impact and costs of the disease; (b) VNS eligibility and clinical results; (c) quality of life (QoL) after VNS therapy; (d) economic impact and productivity regained after VNS; and (e) costs of VNS. Results: Literature data indicate VNS as an effective treatment with a potential positive impact on social aspects and on quality of life. The diagnosis-related group (DRG) financing, both on national and regional levels, does not cover the cost of the medical device. There was an evident insufficient coverage of the DRG compared to the full cost of implanting the device. Conclusions: VNS is a palliative treatment for reducing seizure frequency and intensity. Despite its economic cost, VNS should improve patients' quality of life and reduce care needs.
Keywords: drug-resistant epilepsy; health technology assessment; vagus nerve stimulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Pahl K., de Boer H.M. Atlas: Epilepsy Care in the World. WHO; Geneva, Switzerland: 2005. Epilepsy and Rights; pp. 72–73.
-
- Marras C.E., Canevini M.P., Colicchio G., Guerrini R., Rubboli G., Scerrati M., Spreafico R., Tassi L., Lorusso G., Tinuper P., et al. Health Technology Assessment report on the presurgical evaluation and surgical treatment of drug-resistant epilepsy. Epilepsia. 2013;54:49–58. doi: 10.1111/epi.12309. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical