A Comparative Study of Supervised Machine Learning Algorithms for the Prediction of Long-Range Chromatin Interactions
- PMID: 32847102
- PMCID: PMC7563616
- DOI: 10.3390/genes11090985
A Comparative Study of Supervised Machine Learning Algorithms for the Prediction of Long-Range Chromatin Interactions
Abstract
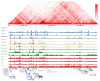
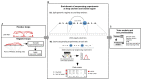
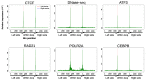
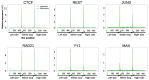
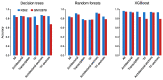
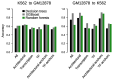
The role of three-dimensional genome organization as a critical regulator of gene expression has become increasingly clear over the last decade. Most of our understanding of this association comes from the study of long range chromatin interaction maps provided by Chromatin Conformation Capture-based techniques, which have greatly improved in recent years. Since these procedures are experimentally laborious and expensive, in silico prediction has emerged as an alternative strategy to generate virtual maps in cell types and conditions for which experimental data of chromatin interactions is not available. Several methods have been based on predictive models trained on one-dimensional (1D) sequencing features, yielding promising results. However, different approaches vary both in the way they model chromatin interactions and in the machine learning-based strategy they rely on, making it challenging to carry out performance comparison of existing methods. In this study, we use publicly available 1D sequencing signals to model cohesin-mediated chromatin interactions in two human cell lines and evaluate the prediction performance of six popular machine learning algorithms: decision trees, random forests, gradient boosting, support vector machines, multi-layer perceptron and deep learning. Our approach accurately predicts long-range interactions and reveals that gradient boosting significantly outperforms the other five methods, yielding accuracies of about 95%. We show that chromatin features in close genomic proximity to the anchors cover most of the predictive information, as has been previously reported. Moreover, we demonstrate that gradient boosting models trained with different subsets of chromatin features, unlike the other methods tested, are able to produce accurate predictions. In this regard, and besides architectural proteins, transcription factors are shown to be highly informative. Our study provides a framework for the systematic prediction of long-range chromatin interactions, identifies gradient boosting as the best suited algorithm for this task and highlights cell-type specific binding of transcription factors at the anchors as important determinants of chromatin wiring mediated by cohesin.
Keywords: chromatin interactions; genome architecture; genomics; machine-learning; prediction.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Rao S.S., Huntley M.H., Durand N.C., Stamenova E.K., Bochkov I.D., Robinson J.T., Sanborn A.L., Machol I., Omer A.D., Lander E.S., et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. - DOI - PMC - PubMed

