Galectins in Intra- and Extracellular Vesicles
- PMID: 32847140
- PMCID: PMC7563435
- DOI: 10.3390/biom10091232
Galectins in Intra- and Extracellular Vesicles
Abstract
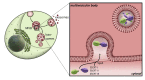
Carbohydrate-binding galectins are expressed in various tissues of multicellular organisms. They are involved in autophagy, cell migration, immune response, inflammation, intracellular transport, and signaling. In recent years, novel roles of galectin-interaction with membrane components have been characterized, which lead to the formation of vesicles with diverse functions. These vesicles are part of intracellular transport pathways, belong to the cellular degradation machinery, or can be released for cell-to-cell communication. Several characteristics of galectins in the lumen or at the membrane of newly formed vesicular structures are discussed in this review and illustrate the need to fully elucidate their contributions at the molecular and structural level.
Keywords: endosome; exosome; galectin; non-classical secretion; sorting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

