Optogenetic stimulation of the motor cortex alleviates neuropathic pain in rats of infraorbital nerve injury with/without CGRP knock-down
- PMID: 32847499
- PMCID: PMC7448516
- DOI: 10.1186/s10194-020-01174-7
Optogenetic stimulation of the motor cortex alleviates neuropathic pain in rats of infraorbital nerve injury with/without CGRP knock-down
Abstract
Background: Previous studies have reported that electrical stimulation of the motor cortex is effective in reducing trigeminal neuropathic pain; however, the effects of optical motor cortex stimulation remain unclear.
Objective: The present study aimed to investigate whether optical stimulation of the primary motor cortex can modulate chronic neuropathic pain in rats with infraorbital nerve constriction injury.
Methods: Animals were randomly divided into a trigeminal neuralgia group, a sham group, and a control group. Trigeminal neuropathic pain was generated via constriction of the infraorbital nerve and animals were treated via selective inhibition of calcitonin gene-related peptide in the trigeminal ganglion. We assessed alterations in behavioral responses in the pre-stimulation, stimulation, and post-stimulation conditions. In vivo extracellular recordings were obtained from the ventral posteromedial nucleus of the thalamus, and viral and α-CGRP expression were investigated in the primary motor cortex and trigeminal ganglion, respectively.
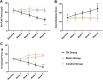
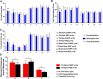
Results: We found that optogenetic stimulation significantly improved pain behaviors in the trigeminal neuralgia animals and it provided more significant improvement with inhibited α-CGRP state than active α-CGRP state. Electrophysiological recordings revealed decreases in abnormal thalamic firing during the stimulation-on condition.
Conclusion: Our findings suggest that optical motor cortex stimulation can alleviate pain behaviors in a rat model of trigeminal neuropathic pain. Transmission of trigeminal pain signals can be modulated via knock-down of α-CGRP and optical motor cortex stimulation.
Keywords: Motor cortex; Neuropathic pain; Optogenetics; Thalamus; Trigeminal ganglion; α-CGRP.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures





References
-
- Henderson JM, Lad SP. Motor cortex stimulation and neuropathic facial pain. Neurosurg Focus. 2006;21(6):1–4. - PubMed
-
- Saavedra LC, Mendonca M, Fregni F. Role of the primary motor cortex in the maintenance and treatment of pain in fibromyalgia. Med Hypotheses. 2014;83(3):332–336. - PubMed
-
- Fontaine D, Hamani C, Lozano A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: critical review of the literature. J Neurosurg. 2009;110(2):251–256. - PubMed
-
- Delavallee M, Rooijakkers H, Koerts G, Raftopoulos C. Motor cortex stimulation in a three-year-old child with trigeminal neuropathic pain caused by a malignant glioma in the cerebellopontine angle: case report. Neurosurgery. 2011;69(2):E494–E4E6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

