Aromatase inhibitor use, side effects and discontinuation rates in gynecologic oncology patients
- PMID: 32847676
- PMCID: PMC8036903
- DOI: 10.1016/j.ygyno.2020.08.015
Aromatase inhibitor use, side effects and discontinuation rates in gynecologic oncology patients
Abstract
Objective: Aromatase inhibitors (AI) are frequently prescribed in gynecologic oncology. We sought to define the frequency and duration of AI use, characterize AI side effects and determine the reasons for discontinuation in these patients.
Methods: Uterine and ovarian cancer patients with AI use for gynecologic cancer therapy were identified retrospectively. Data were abstracted from the electronic medical record, including cancer type, stage, prior cancer treatments, body mass index, concurrent medications, prevalence of AI side effects before and during AI therapy, length of AI treatment and reason for AI discontinuation.
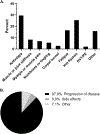
Results: 146 women received AI therapy, with 68 for ovarian cancer (46.6%) and 78 for uterine cancer (53.4%). The majority (71.9%) had advanced stage disease at diagnosis. 54.1% noted AI-associated side effects within the first three visits after starting AI therapy. The most common side effects were arthralgias (29.5%), hot flashes (25.3%), new/worsening fatigue (16.4%), muscle or joint stiffness (8.2%) and myalgias (6.8%). The mean duration of therapy was 14.7 months. Gabapentin or selective serotonin reuptake inhibitor (SSRI) use was associated with decreased musculoskeletal side effects (gabapentin: p < .001, OR 0.88, 95% CI 0.83-0.94; SSRI: p < .001, OR 0.82, 95% CI 0.77-0.89). The most common reason for AI discontinuation was disease progression (87.9%), with 5.0% discontinuing due to side effects and 7.1% for other reasons.
Conclusion: AI therapy for gynecologic cancers is frequently associated with musculoskeletal side effects, but rarely leads to treatment discontinuation. Thus, AI side effects should be assessed in gynecologic cancer patients to allow potential mitigation of symptoms through adjunct therapies.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors report no conflicts of interest.
Figures
Similar articles
-
Online discussion of drug side effects and discontinuation among breast cancer survivors.Pharmacoepidemiol Drug Saf. 2013 Mar;22(3):256-62. doi: 10.1002/pds.3365. Epub 2013 Jan 16. Pharmacoepidemiol Drug Saf. 2013. PMID: 23322591 Free PMC article.
-
Aromatase inhibitor-associated arthralgia syndrome.Breast. 2007 Jun;16(3):223-34. doi: 10.1016/j.breast.2007.01.011. Epub 2007 Mar 21. Breast. 2007. PMID: 17368903 Review.
-
Effect of Aromatase Inhibitor Therapy on Sleep and Activity Patterns in Early-stage Breast Cancer.Clin Breast Cancer. 2018 Apr;18(2):168-174.e2. doi: 10.1016/j.clbc.2017.12.012. Epub 2017 Dec 27. Clin Breast Cancer. 2018. PMID: 29361424 Free PMC article.
-
Side effects of aromatase inhibitors versus tamoxifen: the patients' perspective.Am J Surg. 2006 Oct;192(4):496-8. doi: 10.1016/j.amjsurg.2006.06.018. Am J Surg. 2006. PMID: 16978958
-
Aromatase inhibitor therapy: toxicities and management strategies in the treatment of postmenopausal women with hormone-sensitive early breast cancer.Breast Cancer Res Treat. 2011 Apr;126(2):295-310. doi: 10.1007/s10549-011-1351-3. Epub 2011 Jan 20. Breast Cancer Res Treat. 2011. PMID: 21249443 Review.
Cited by
-
Characterization of folic acid-functionalized PLA-PEG nanomicelle to deliver Letrozole: A nanoinformatics study.IET Nanobiotechnol. 2022 Jun;16(4):103-114. doi: 10.1049/nbt2.12073. Epub 2021 Nov 23. IET Nanobiotechnol. 2022. PMID: 34812575 Free PMC article.
-
Two natural products from the seeds of Citrus reticulata Blanco (Rutaceae) inhibit estrogen biosynthesis by regulating the PI3K-aromatase pathway.Front Pharmacol. 2025 Jun 12;16:1583409. doi: 10.3389/fphar.2025.1583409. eCollection 2025. Front Pharmacol. 2025. PMID: 40575782 Free PMC article.
-
Impact of risk and lifestyle factors on therapy goals in the treatment of breast cancer and gynecological cancer patients with integrative medicine.Arch Gynecol Obstet. 2025 Jun;311(6):1683-1695. doi: 10.1007/s00404-025-08002-w. Epub 2025 Apr 9. Arch Gynecol Obstet. 2025. PMID: 40204922 Free PMC article.
-
Aromatase inhibitors as a therapeutic strategy for male prolactinoma resistant to dopamine agonists: a retrospective cohort study and literature review.J Endocrinol Invest. 2024 May;47(5):1295-1303. doi: 10.1007/s40618-023-02231-z. Epub 2023 Nov 8. J Endocrinol Invest. 2024. PMID: 37938428 Review.
-
Polypharmacy, over-the-counter medications, and aromatase inhibitor adherence in early-stage breast cancer.Breast Cancer Res Treat. 2024 Apr;204(3):539-546. doi: 10.1007/s10549-023-07218-1. Epub 2024 Jan 10. Breast Cancer Res Treat. 2024. PMID: 38198070 Free PMC article. Clinical Trial.
References
-
- Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al., Adjuvant endocrine therapy for women with hormone receptor—positive breast Cancer: American Society of Clinical Oncology clinical practice guideline focused update, J. Clin. Oncol. 32 (2014) 2255–2269, 10.1200/jco.2013.54.2258. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

