Potential impact of missing outcome data on treatment effects in systematic reviews: imputation study
- PMID: 32847800
- PMCID: PMC7448113
- DOI: 10.1136/bmj.m2898
Potential impact of missing outcome data on treatment effects in systematic reviews: imputation study
Erratum in
-
Potential impact of missing outcome data on treatment effects in systematic reviews: imputation study.BMJ. 2020 Dec 14;371:m4783. doi: 10.1136/bmj.m4783. BMJ. 2020. PMID: 33318027 No abstract available.
Abstract
Objective: To assess the risk of bias associated with missing outcome data in systematic reviews.
Design: Imputation study.
Setting: Systematic reviews.
Population: 100 systematic reviews that included a group level meta-analysis with a statistically significant effect on a patient important dichotomous efficacy outcome.
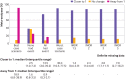
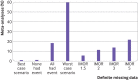
Main outcome measures: Median percentage change in the relative effect estimate when applying each of the following assumption (four commonly discussed but implausible assumptions (best case scenario, none had the event, all had the event, and worst case scenario) and four plausible assumptions for missing data based on the informative missingness odds ratio (IMOR) approach (IMOR 1.5 (least stringent), IMOR 2, IMOR 3, IMOR 5 (most stringent)); percentage of meta-analyses that crossed the threshold of the null effect for each method; and percentage of meta-analyses that qualitatively changed direction of effect for each method. Sensitivity analyses based on the eight different methods of handling missing data were conducted.
Results: 100 systematic reviews with 653 randomised controlled trials were included. When applying the implausible but commonly discussed assumptions, the median change in the relative effect estimate varied from 0% to 30.4%. The percentage of meta-analyses crossing the threshold of the null effect varied from 1% (best case scenario) to 60% (worst case scenario), and 26% changed direction with the worst case scenario. When applying the plausible assumptions, the median percentage change in relative effect estimate varied from 1.4% to 7.0%. The percentage of meta-analyses crossing the threshold of the null effect varied from 6% (IMOR 1.5) to 22% (IMOR 5) of meta-analyses, and 2% changed direction with the most stringent (IMOR 5).
Conclusion: Even when applying plausible assumptions to the outcomes of participants with definite missing data, the average change in pooled relative effect estimate is substantive, and almost a quarter (22%) of meta-analyses crossed the threshold of the null effect. Systematic review authors should present the potential impact of missing outcome data on their effect estimates and use this to inform their overall GRADE (grading of recommendations assessment, development, and evaluation) ratings of risk of bias and their interpretation of the results.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interest: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Cochrane Methods Innovation Fund for the submitted work, no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
-
- National Research Council The Prevention and Treatment of Missing Data in Clinical Trials. The National Academic Press, 2010. - PubMed
-
- Hussain JA, Bland M, Langan D, Johnson MJ, Currow DC, White IR. Quality of missing data reporting and handling in palliative care trials demonstrates that further development of the CONSORT statement is required: a systematic review. J Clin Epidemiol 2017;88:81-91. 10.1016/j.jclinepi.2017.05.009 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
