Oncological care organisation during COVID-19 outbreak
- PMID: 32847836
- PMCID: PMC7451457
- DOI: 10.1136/esmoopen-2020-000853
Oncological care organisation during COVID-19 outbreak
Erratum in
-
Correction: Oncological care organisation during COVID-19 outbreak.ESMO Open. 2020 Oct;5(5):e000682corr1. doi: 10.1136/esmoopen-2020-000853corr1. ESMO Open. 2020. PMID: 33093021 Free PMC article. No abstract available.
Abstract
Background: COVID-19 appeared in late 2019, causing a pandemic spread. This led to a reorganisation of oncology care in order to reduce the risk of spreading infection between patients and healthcare staff. Here we analysed measures taken in major oncological units in Europe and the USA.
Methods: A 46-item survey was sent by email to representatives of 30 oncological centres in 12 of the most affected countries. The survey inquired about preventive measures established to reduce virus spread, patient education and processes employed for risk reduction in each oncological unit.
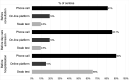
Results: Investigators from 21 centres in 10 countries answered the survey between 10 April and 6 May 2020. A triage for patients with cancer before hospital or clinic visits was conducted by 90.5% of centres before consultations, 95.2% before day care admissions and in 100% of the cases before overnight hospitalisation by means of phone calls, interactive online platforms, swab test and/or chest CT scan. Permission for caregivers to attend clinic visits was limited in many centres, with some exceptions (ie, for non-autonomous patients, in the case of a new diagnosis, when bad news was expected and for terminally ill patients). With a variable delay period, the use of personal protective equipment was unanimously mandatory, and in many centres, only targeted clinical and instrumental examinations were performed. Telemedicine was implemented in 76.2% of the centres. Separated pathways for COVID-19-positive and COVID-19-negative patients were organised, with separate inpatient units and day care areas. Self-isolation was required for COVID-19-positive or symptomatic staff, while return to work policies required a negative swab test in 76.2% of the centres.
Conclusion: Many pragmatic measures have been quickly implemented to deal with the health emergency linked to COVID-19, although the relative efficacy of each intervention should be further analysed in large observational studies.
Keywords: COVID-19; oncological care.
© Author (s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. Published by BMJ on behalf of the European Society for Medical Oncology.
Conflict of interest statement
Competing interests: HSR reported fundings to the Institution from Eisai, Genentech, Lilly, Macrogenics, Merk, Novartis, OBI Pharma, Odonate Therapeutics, Immunomedics, Daiichi-Sankyo and Pfizer; travel fees from Pfizer, Novartis, MacroGenics, Mylan, Daiichi-Sankyo and AstraZeneca; consulting roles for Samsung and Celtrion. MM reported personal fees from Roche, Novartis, Puma, AstraZeneca, Amgen, Taiho Oncology, Daiichi-Sankyo, PharmaMar, Eli-Lilly and Pfizer; grants from Roche, Novartis and Puma. MC reported a grant from Pfizer; personal fees from CytoDyn, Pfizer, Eli-Lilly, Novartis, Foundation Medicine, G1 Therapeutics, Sermionex and Genentech. JC reported funding to the institution from Roche, Ariad Pharmaceuticals, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer healthcare, Eisai, F. Hoffman-La Roche, Guardanth health, Merck Sharp & Dohme, Pfizer, Piqur Therapeutics, Puma C and Queen Mary University of London; honoraria from Roche, Novartis, Celgene, Eisai, Pfizer, Samsung Bioepis, Eli-Lilly, Merck Sharp & Dohme, and Daiichi Sankyo; travel fees from Roche, Novartis, Eisai, Pfizer, Daiichi Sankyo; consulting/advisor role for Roche, Celgene, Cellestia, AstraZeneca, Biothera Pharmaceutical, Merus, Seattle Genetics, Daiichi-Sankyo, Erytech, Athenex, Polyphor, Lilly, Servier, Merck Sharp&Dohme, GSK, Leuko, Bioasis, Clovis Oncology and Boehringer Ingelheim; stock, patents and intellectual property of MedSIR. SAH reported grants from Ambrx, Amgen, Arvinas, Bayer, Daiichi-Sankyo, Genentech/Roche, GSK, Immunomedics, Eli-Lilly, Macrogenics, Novartis, Pfizer, OBI Pharma, Pieris, Puma, Radius, Sanofi, Seattle Genetics and Dignitana; travel fees from Lilly and Novartis; stock options from NK Max; editorial support from Roche/Genetech, Eli-Lilly and Pfizer. MT declares travel, accomodations, expenses by Roche, Bristol-Myers Squibb, Astra Zeneca, Takeda; activity as medical writer supported by Novartis and Amgen, outside the present work. MC reported funding to the institution from Sanofi, Pierre-Fabre, AstraZeneca, Servier, Novartis, Abbvie, Accord and Pfizer; personal fees from GT1, Sanofi, Pierre-Fabre, AstraZeneca, Servier, Novartis, Abbvie, Accord and Pfizer. RB reported personal fees from Accord, AstraZeneca, Daiichi-Sankyo, Eisai, Eli-Lilly, MSD, Novartis, Roche, Puma, Pierre-Fabre and Sandoz; grants from Daiichi-Sankyo, Novartis and Roche; non-financial support from Eli-Lilly and MSD. SDP reported personal fees from Roche, Novartis, Pfizer, Celgene, AstraZeneca and Eli-Lilly. VM reported funding to the institution from Novartis, Roche, Seattle Genetics and Genentech; personal fees from Amgen, AstraZeneca, Daiichi-Sankyo, Eisai, Pfizer, MSD, Novartis, Roche, Teva and Seattle Genetics; consultancy honoraria from Genomic Health, Hexal, Roche, Pierre-Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi-Sankyo, Eisai, Eli-Lilly, Tesaro, Seattle Genetics and Nektar. J-PM reported personal fees from Roche, AstraZeneca, Bayer, Innate, Merck-Serono, Boeringher, BMS, Novartis, Janssen, Incyte, Cue Biopharma, ALX Oncology and Pfizer; grant from BMS; travel fee from Amgen; other support from MSD, Psioxus, Debio and Nanobiotix. GJ reported personal fees from Novartis, Roche, Pfizer, Eli-Lilly, Amgen, BMS, AstraZeneca, Daiichi-Sankyo and Abbvie; grants from Novartis, Roche and Pfizer; non-financial support from Medimmune and Merck KGaA.
Figures



References
-
- COVID-19 data Repository by the center for systems science and engineering (CSSE) at Johns Hopkins University. Available: https://github.com/CSSEGISandData/COVID-19
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

