The Dynamic Interface of Viruses with STATs
- PMID: 32847860
- PMCID: PMC7592203
- DOI: 10.1128/JVI.00856-20
The Dynamic Interface of Viruses with STATs
Abstract
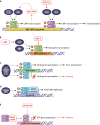
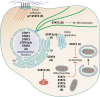
Viruses commonly antagonize the antiviral type I interferon response by targeting signal transducer and activator of transcription 1 (STAT1) and STAT2, key mediators of interferon signaling. Other STAT family members mediate signaling by diverse cytokines important to infection, but their relationship with viruses is more complex. Importantly, virus-STAT interaction can be antagonistic or stimulatory depending on diverse viral and cellular factors. While STAT antagonism can suppress immune pathways, many viruses promote activation of specific STATs to support viral gene expression and/or produce cellular conditions conducive to infection. It is also becoming increasingly clear that viruses can hijack noncanonical STAT functions to benefit infection. For a number of viruses, STAT function is dynamically modulated through infection as requirements for replication change. Given the critical role of STATs in infection by diverse viruses, the virus-STAT interface is an attractive target for the development of antivirals and live-attenuated viral vaccines. Here, we review current understanding of the complex and dynamic virus-STAT interface and discuss how this relationship might be harnessed for medical applications.
Keywords: STAT signaling; STAT transcription factors; host-pathogen interactions; immune evasion; virus-host interactions.
Copyright © 2020 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

